Question
In: Chemistry
8.03 x 10^23 moleucles of oxygen react with excess nitrogen. what is the maximum number of...
8.03 x 10^23 moleucles of oxygen react with excess nitrogen. what is the maximum number of grams of dinitrogen tetroxide that can be produced?
42 grams of cyclohexane burns in excess air to form carbon dioxide and water. How many grams of carbon dioxide and of water vapor are produced?
Solutions
Expert Solution
We know that 1 mole of any substance contains 6.023x10 23 ( avagadro number ) molecules
1 mole of O2 contains 6.023x10 23 molecules
Z moles of O2 contains 8.03x10 23 molecules
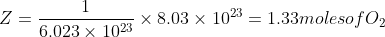
The balanced equation
is 2O2 +
N2 N2O4
N2O4
According to the above balanced equation ,
2 moles of O2 produces 1 mole of N2O4
1.33 moles of O2 produces M mole of N2O4
Molar mass of N2O4 is = (2x14) + (4x16) = 92 g/mol
So mass of N2O4 produced is , m = Number of moles x molarmass
= 0.665 mol x 92 g/mol
= 61.2 g
------------------------------------------------------------------------------------------------------------------------------------------
The balanced equation is
C6H12 + 9O2 6CO2 + 6H2O
6CO2 + 6H2O
Molar mass of C6H12 is = (6x12) + (12x1) = 84 g/mol
Molar mass of CO2 is = 12 + (2x16) = 44 g/mol
Molar mass of H2O is = (2x1) + 16 = 18 g/mol
According to the balanced equation ,
84 g of C6H12 produces 6x44 g of CO2
84/2 = 42 g of C6H12 produces (6x44) / 2 = 132 g of CO2
From the balanced equation ,
84 g of C6H12 produces 6x18 g of H2O
84/2 = 42 g of C6H12 produces (6x18) / 2 = 54 g of H2O
Related Solutions
When 5.000 grams of ammonia react with an excess of oxygen and CH4 in a bomb...
Nitrogen and oxygen do not react appreciably at room temperature, as illustrated by our atmosphere. But...
Nitrogen and oxygen do not react appreciably at room temperature, as illustrated by our atmosphere. But...
Hydrazine, N2H4, may react with oxygen to form nitrogen gas andwater. If 2.15g of N2H4...
Calculate the work (in kJ) when 2.70 moles of methane react with excess oxygen at 364...
Calculate the work (in kJ) when 2.60 moles of methane react with excess oxygen at 363...
Ammonia gas will react with oxygen gas to yield nitrogen monoxide gas and water vapor. write...
For the following reaction, 9.45 grams of nitrogen monoxide are allowed to react with 8.22 grams of oxygen gas ....
For the following reaction, 10.1 grams of nitrogen gas are allowed to react with 5.97 grams of oxygen gas ....
Determine the number of moles in 4.21 x 10^23 molecules of CaCl2
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
 queen_honey_blossom answered 2 years ago
queen_honey_blossom answered 2 years ago