Question
In: Physics
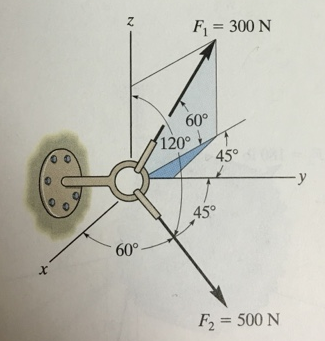
The screw eye is subjected to the two forces shown. Express each force in Cartesian vector form and then determine the resultant force
The screw eye is subjected to the two forces shown. Express each force in Cartesian vector form and then determine the resultant force. Find the magnitude and coordinate direction angles of the resultant force.
Solutions
Related Solutions
Specify the coordinate direction angles of F1 and F2 and express each force as a Cartesian vector.
Specify the coordinate direction angles of F1 and F2 and express each force as a Cartesian vector. State the magnitude and coordinate direction angles of the resultant vector.
To find the resultant of multiple forces using Cartesian components and to determine the direction of...
To find the resultant of multiple forces using Cartesian
components and to determine the direction of this resultant from
its components. As shown, three forces act on the tip of a pole. F1
= ( -60 i + 145 j + 45 k ) N. F2 = 140 N and forms the following
angles with the x, y, and z axes, respectively: α = 55.2 ∘ , β =
65.6 ∘ , and γ = 135.2 ∘ . F3 =...
Determine the resultant vector D when the three vectors below are added together. Express your result...
Determine the resultant vector D when the three
vectors below are added together. Express your result in a
magnitude and direction.
A = 5kN@40*
B = 10kN@140*
C = 25kN@240*
Two forces of 55N and 85N act on an object simultaneously and the resultant force is 125N. What is the measurement of the angle between the two forces?
Two forces of 55N and 85N act on an object simultaneously and the resultant force is 125N. What is the measurement of the angle between the two forces?
Forces F1 and F2 act on the bracket as shown. Determine the projection Fb of their resultant R onto the b-axis.
Forces F1 and F2 act on the bracket as shown. Determine the projection Fb of their resultant R onto the b-axis.
The rigid structural member is subjected to a couple consisting of the two 100-N forces. Replace this couple by an equivalent couple consisting of the two forces P and -P, each of which has a magnitude of 400 N. Determine the proper angle θ.
The rigid structural member is subjected to a couple consisting of the two 100-N forces. Replace this couple by an equivalent couple consisting of the two forces P and -P, each of which has a magnitude of 400 N. Determine the proper angle θ.
The 500-N force F is applied to the vertical pole as shown 1. Determine the scalar components of the force vector F along the x'- and y'-axes. 2. Determine the scalar components of F along the x- and y'-axes
The 500-N force F is applied to the vertical pole as shown1. Determine the scalar components of the force vector F along the x'- and y'-axes. 2. Determine the scalar components of F along the x- and y'-axes.
1)Determine the pH of each solution. Part A 0.20 M KCHO2 Express your answer to two...
1)Determine the pH of each solution.
Part A
0.20 M KCHO2
Express your answer to two decimal places.
Part B
0.21 M CH3NH3I
Express your answer to two decimal places.
Part C
0.22 M KI
Express your answer to two decimal places.
2)
Part A
Rank the following compounds in order of decreasing acid
strength using periodic trends.
Rank the acids from strongest to weakest. To rank items as
equivalent, overlap them.
LiH, H2O, HCl, HBr
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
ADVERTISEMENT


 genius_generous answered 3 years ago
genius_generous answered 3 years ago