Question
In: Chemistry
For the Stern-Volmer, determine the condition when the molar concentration [Q] is equal to 1/kq
For the Stern-Volmer, determine the condition when the molar concentration [Q] is equal to 1/kq
Solutions
Expert Solution
Answer: According to the question , Here the equation is look like as :

where A is one chemical species, Q is another (known as a quencher) and * designates an excited state.
The kinetics of this process follows the Stern–Volmer relationship:
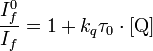
Now , Here the conditions is
[Q]Kq = [I0f /If -1] /T0 is become constant then only the concentration of Q is become equal to the 1/kq .
Hence it is all about the given question . Thank you :)
Related Solutions
Determine the concentration of Cl- remaining in solution if a precipitate forms when equal volumes of...
Determine the concentration of Cl- remaining in solution if a
precipitate forms when equal volumes of 7.76 x 10^-3 M HCl and 5.24
x10^-7 M AgNO3 are mixed to produce AgCl. Ksp AgCl = 1.6 x10^-10.
Report the concentration of Cl- as a -log value, pCl = -log Cl-
concentration
A) Calculate the molar absorptivity constant at each concentration for all wavelengths B) Determine which wavelength...
A) Calculate the molar absorptivity constant at each
concentration for all wavelengths
B) Determine which wavelength you would use to run a calibration
curve for the sample and defend the answer
C) Explain why there are differences at each wavelength (if
there are any) for the calculated molar absorptivity constant
Path length for all exps = 2.5 cm
Concentration
Abs @ 315 nm
Abs @ 320 nm
Abs @ 325 nm
Abs @ 330 nm
Abs @ 335 nm
50...
Given: Molar Concentration of Fe(NO3)3 is 0.2 Molar concentration of NaSCN is 0.001 Volume of Fe(NO3)3...
Given:
Molar Concentration of Fe(NO3)3 is 0.2
Molar concentration of NaSCN is 0.001
Volume of Fe(NO3)3 is 10.00 ml (in 0.1 M HNO3)
Volume of NaSCN is 2.00 ml (in 0.1 M HNO3)
10.00 ml Fe(NO3)3 + 2.00ml NaSCN + 13.00 ml HNO3= 25 mL
Calculate:
a) Moles of SCN-
b) [SCN-] (25.0 ml)
c) [FeNCS2+]
How could an experiment such as that performed by Stern and Gerlach be used to determine...
How could an experiment such as that performed by Stern and
Gerlach be used to determine the total angular momentum quantum
number of the ground state of a given atom or ion? [magnetic
sub-levels, number of spots…]
1. What is the molar concentration of sucrose in a can of Red Bull (27 g...
1. What is the molar concentration of sucrose in a can of Red
Bull (27 g sucrose/250 mL)?
2. How much glycine is needed to make 125 mL of a 1.5 M stock
solution?
3. What volume of ethylene glycol is needed to prepare 200 mL of
a 0.25 M stock solution?
4. Using the above stock solutions from questions #2 and #3, how
would you prepare 750 mL of a solution that is 10 mM in glycine and
25...
1) a. What is the molar concentration (i.e., molarity) of sucrose (C12H22O11) if 150.0 g is...
1) a. What is the molar concentration (i.e., molarity) of
sucrose (C12H22O11) if 150.0 g is dissolved in 250.0 mL of
solution?
b.What is the molar concentration of methanol (CH3OH) if 125.0
mL is dissolved in enough water to make 15.0 L of solution? The
density of methanol is 0.792 g/mL.
c.How many grams of NaOH are contained in 250.0 mL of a 0.400 M
sodium hydroxide solution?
d.The drinking water standard for lead is 15 ppb (parts per
billion)....
Calculate the molar concentration of OH negative in water solutions with the following H3O positive molar...
Calculate the molar concentration of OH negative in water
solutions with the following H3O positive molar concentrations:
a) 0.044
b) 1.3 * 10 to the negative 4 power
c) 0.0087
d) 7.9 * 10 to the negative 10 power
e) 3.3 * 10 to the negative 2 power
(Please show clear handwriting and step by step) Thank you!!
calculate the mass concentration and molar concentration for 67.2% (by weight) nitric acid solution. The density...
calculate the
mass concentration and molar concentration for 67.2% (by weight)
nitric acid solution. The density of nitric acid solution (67.2% by
weight) is 1.4 g/cm
1) The equation A=Ebc directly relates the concentration of a sample, its molar absorptivity (E), and...
1) The equation A=Ebc directly relates the concentration of a
sample, its molar absorptivity (E), and the absorbance of a sample.
Instead of bothering with a standard curve, why not make two
measurements: first, measure the absorbance of a sample of known
concentration in order to compute the molar absorptivity. Second,
measure the absorbance of the solution with unknown concentration
and use the molar absorptivity from the first to calculate the
concentration. Explain why this would, or would not, be...
1. At 25°C, 298.25 mL of an aqueous solution of NiCl2 with a molar analytical concentration...
1. At 25°C, 298.25 mL of an
aqueous solution of NiCl2 with a molar analytical concentration of
0.00004873 M is added to 487.37 mL of an aqueous solution of
AlBr3with a molar analytical concentration of 0.00005000
M.
What is the equilibrium concentration of Ni2+ in the resulting
solution?
2. What is the equilibrium concentration of Al3+ in the
resulting solution?
3. What is the ionic strength of the resulting solution?
4. What is the activity coefficient of Ni2+ in
the...
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
ADVERTISEMENT
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago