Question
In: Chemistry
Using molecular deuterium in the presence of the metal catalyst palladium hydrogenate this alkene: 2,3-dimethylbut-1-ene
Using molecular deuterium in the presence of the metal catalyst palladium hydrogenate this alkene: 2,3-dimethylbut-1-ene
Solutions
Expert Solution
Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst.
Substrate
The addition of H2 to an alkene affords an alkane in the prototypical reaction:
RCH=CH2 + H2 ? RCH2CH3 (R = alkyl, aryl)
Hydrogenation is sensitive to steric hindrance explaining the selectivity for reaction with the exocyclic double bond but not the internal double bond.
An illustrative example of a hydrogenation reaction is the addition of hydrogen to maleic acid to form succinic acid. Numerous important applications of this petrochemical are found in pharmaceutical and food industries.
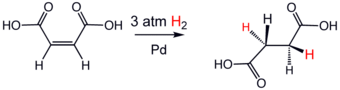
An important characteristic of alkene and alkyne hydrogenations, both the homogeneously and heterogeneously catalyzed versions, is that hydrogen addition occurs with "syn addition", with hydrogen entering from the least hindered side. Typical substrates are listed in the table
| substrate | product | comments |
| alkene, R2C=CR'2 | alkane, R2CHCHR'2 | many catalysts, one application is margarine |
| alkyne, RCCR | alkene, cis-RHC=CHR' | over-hydrogenation to alkane can be problematic |
| aldehyde, RCHO | primary alcohol, RCH2OH | easy substrate |
| ketone, R2CO | secondary alcohol, R2CHOH | more challenging than RCHO, prochiral for unsymmetrical ketones |
| ester, RCO2R' | two alcohols, RCH2OH + R'OH | challenging substrate |
| imine, RR'CNR" | amine, RR'CHNHR" | easy substrate, often use transfer hydrogenation, actual precursor is N-protonated |
| amide, RC(O)NR'2 | amine, RCH2NR'2 | challenging substrate |
| nitrile, RCN | primary amine, RCH2NH2 | product amine reactive toward precursor nitrile in some cases |
| nitro, RNO2 | amine, RNH2 | commercial applications use heterogeneous Ni and Ru catalysts; major application is aniline |
Catalysts
With rare exceptions, no reaction below 480
Related Solutions
Using The Degree of Advancement Variable Methane and oxygen react in the presence of a catalyst...
1. What effect does the presence of metal cations have on the color of a solution...
1.Write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver...
Two catalysts are being considered for a chemical process. Sixteen batches were completed using Catalyst 1,...
Two catalysts are being considered for a chemical process. Sixteen batches were completed using Catalyst 1,...
Experiment 16: Synthesis of Fragrant Esters (using carboxylic acid and alcohol w/ sulfuric acid catalyst) 1)...
Using Apple ... 1. Describe the methods that you plan to use to create global presence...
1. Using your knowledge of cell & molecular biology techniques, design an experiment to investigate a...
1. The thermite reaction is performed using 8.6g Fe2O3 and 1.8g powdered Al metal/ a. Which...
Using molecular structure, explain why tetracosane has a higher melting point than 1-tetradecanol despite the fact...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...
- How many grays is this?Part A A dose of 4.7 Sv of γ rays in a...
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago