Question
In: Physics
The absolute zero temperature is equal to 0 k or -273.15. Why we cannot create objects...
The absolute zero temperature is equal to 0 k or -273.15. Why we cannot create objects with even lower temperature than 0k? Why the absolute zero temperature exists? What happens ( on microscopic scale) to atoms and molecules when their temperature is near the absolute zero?
Solutions
Expert Solution
the temperature of a body at rest is a measure of the mean of
the energy of the translational, vibrational and rotational motions
of matter's particle constituents, such as molecules, atoms, and
subatomic particles. The full variety of these kinetic motions,
along with potential energies of particles, and also occasionally
certain other types of particle energy in equilibrium with these,
make up the total internal energy of a substance. Internal energy
is loosely called the heat energy or thermal energy in conditions
when no work is done upon the substance by its surroundings, or by
the substance upon the surroundings. Internal energy may be stored
in a number of ways within a substance, each way constituting a
"degree of freedom". At equilibrium, each degree of freedom will
have on average the same energy: 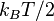 where
where 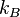 is the Boltzmann constant, unless that degree of freedom is in the
quantum regime. The internal degrees of freedom (rotation,
vibration, etc.) may be in the quantum regime at room temperature,
but the translational degrees of freedom will be in the classical
regime except at extremely low temperatures (fractions of kelvins)
and it may be said that, for most situations, the thermodynamic
temperature is specified by the average translational kinetic
energy of the particles.
is the Boltzmann constant, unless that degree of freedom is in the
quantum regime. The internal degrees of freedom (rotation,
vibration, etc.) may be in the quantum regime at room temperature,
but the translational degrees of freedom will be in the classical
regime except at extremely low temperatures (fractions of kelvins)
and it may be said that, for most situations, the thermodynamic
temperature is specified by the average translational kinetic
energy of the particles.
As a substance cools, different forms of internal energy and their related effects simultaneously decrease in magnitude: the latent heat of available phase transitions are liberated as a substance changes from a less ordered state to a more ordered state; the translational motions of atoms and molecules diminish (their kinetic temperature decreases); the internal motions of molecules diminish (their internal temperature decreases); conduction electrons (if the substance is an electrical conductor) travel somewhat slower;[30] and black-body radiation's peak emittance wavelength increases (the photons' energy decreases). When the particles of a substance are as close as possible to complete rest and retain only ZPE-induced quantum mechanical motion, the substance is at the temperature of absolute zero (T=0).
Note that whereas absolute zero is the point of zero thermodynamic temperature and is also the point at which the particle constituents of matter have minimal motion, absolute zero is not necessarily the point at which a substance contains zero thermal energy; one must be very precise with what one means by internal energy. Often, all the phase changes that can occur in a substance, will have occurred by the time it reaches absolute zero. However, this is not always the case. Notably, T=0 helium remains liquid at room pressure and must be under a pressure of at least 25 bar (2.5 MPa) to crystallize. This is because helium's heat of fusion (the energy required to melt helium ice) is so low (only 21 joules per mole) that the motion-inducing effect of zero-point energy is sufficient to prevent it from freezing at lower pressures. Only if under at least 25 bar (2.5 MPa) of pressure will this latent thermal energy be liberated as helium freezes while approaching absolute zero. A further complication is that many solids change their crystal structure to more compact arrangements at extremely high pressures (up to millions of bars, or hundreds of gigapascals). These are known as solid-solid phase transitions wherein latent heat is liberated as a crystal lattice changes to a more thermodynamically favorable, compact one.
The above complexities make for rather cumbersome blanket statements regarding the internal energy in T=0 substances. Regardless of pressure though, what can be said is that at absolute zero, all solids with a lowest-energy crystal lattice such those with a closest-packed arrangement (see Fig. 8, above left) contain minimal internal energy, retaining only that due to the ever-present background of zero-point energy.[3][31] One can also say that for a given substance at constant pressure, absolute zero is the point of lowest enthalpy (a measure of work potential that takes internal energy, pressure, and volume into consideration).[32] Lastly, it is always true to say that all T=0 substances contain zero kinetic thermal energy.[3][7]
Related Solutions
Is a p-value could be equal to zero (0)?
Thermal energy ~ kT , where T is the absolute temperature (Kelvin) and k is Boltzmann's...
The velocity of sound, v (m/s), increases as the square rootof the absolute temperature, T (K):...
Is there any free electron or hole in silicon crystal at absolute zero (0K)? Why? Is...
Prove that if {xn} converges to x does not equal to 0 and xn is non-zero...
Given an array of integers, and given a specific value k (not equal to 0), produce...
Why studying synaptic plasticity (LTP and LTD) cannot be equal to studying memory?
Explain speed of light, and why, when we look at celestial objects, we are not seeing...
Why we cannot print money to pay back the debt
Briefly explain the significance of the Kelvin scale for temperature. What is special about 0 K?
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...
- How many grays is this?Part A A dose of 4.7 Sv of γ rays in a...
 genius_generous answered 3 years ago
genius_generous answered 3 years ago