Question
In: Chemistry
Which statement is NOT correct? A. You can increase entropy and still have a spontaneous reaction....
Which statement is NOT correct?
A. You can increase entropy and still have a spontaneous reaction.
B. If there is a decrease in the energy of the system, the reaction is spontaneous.
C. Spontaneous reactions will tend to maximize entropy and minimize enthalpy at given temperature
D. Entropy can drive a reaction that is strongly endothermic
Solutions
Expert Solution
For a process that occurs at constant temperature and pressure, spontaneity can be determined using the change in Gibbs free energy, which is given by:
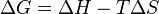 ,
,
In cases where ΔG is:
- negative, the process is spontaneous and may proceed in the forward direction as written.
This set of rules can be used to determine four distinct cases by examining the signs of the ΔS and ΔH.
- When ΔS > 0 and ΔH < 0, the process is always spontaneous as written.
- When ΔS < 0 and ΔH > 0, the process is never spontaneous, but the reverse process is always spontaneous.
- When ΔS > 0 and ΔH > 0, the process will be spontaneous at high temperatures and non-spontaneous at low temperatures.
- When ΔS < 0 and ΔH < 0, the process will be spontaneous at low temperatures and non-spontaneous at high temperatures.
So as per this the only statement which is not true is
D. Entropy can drive a reaction that is strongly endothermic
because when a system is strongly endothermic meaning ΔH ha a large +ve value inclreaseing entropy will not make it positive.
Related Solutions
For which of the following reactions would the entropy of reaction (ΔS°rxn) be positive (an increase...
Which statement is NOT correct? A. In an endothermic reaction, the activation energy is usually greater...
3. Which of the following is not correct regarding a prepotential: it is a spontaneous depolarization...
Describe a process in which entropy appears to decrease, but is coupled with an increase in...
Describe a process in which entropy appears to decrease, but is coupled with an increase in...
Which of the following changes leads to an increase in entropy of the system? The evaporation...
determine which of the following pairs of reactants will result in a spontaneous reaction at 25...
What can be said about an Endothermic reaction with a negative entropy change?
Which of the following statement is correct?
Which statement is correct? Select one: A. Firms prefer to increase processing delay on disbursements. B....
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago