Question
In: Chemistry
Draw the structure of XeOF4
Draw the structure of XeOF4.
Solutions
Expert Solution
By using the following steps we can draw the electron dot structures of the given molecules.
Step: 1 Count the total number of valence electrons.
Step: 2 draw the lines to represent the bonds.
Step: 3 Subtract the number of valence electrons used for bonding from the total number
calculated in step 1 to find the number that remains. Assign as many of these remaining
electrons as necessary to the terminal atoms so that each has an octet.
Step: 4 if unassigned electrons remain after step 3 place them central atom.
Step: 5 if unassigned electrons remain after step 3 but the central atom does not yet have an octet, use one or more lone pairs of electrons from a neighboring atom to form a multiple bonds.
The electron dot structure of \(\mathrm{XeOF}_{4}\) has:
Total number of valence electrons
\(=8 e^{-}(\) For \(\mathrm{Xe})+4 \times 7 e^{-}(\) for \(\mathrm{F})+1 \times 6 e^{-}(\) for \(\mathrm{O})\)
\(=42 \mathrm{e}^{-}\)
Therefore,
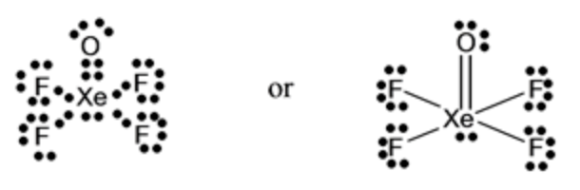
Related Solutions
Draw the Lewis structure for AsF3 Draw the Lewis dot structure. To change the symbol of...
Draw the structure of 2,3-dimethylbutane
Why is ubiquinone aka CoQ called that? Draw its structure and then draw its reduced structure....
Draw a Lewis structure for PO4 3
draw the best Lewis structure for OCl2.
draw the lewis structure for CH3CHBrCHBrCH2CH3 and for CH3CHFCH2OH
Draw the Lewis structure for the I−3.
Draw a Lewis structure, condensed structure and skeletal structure for a compound with molecular formula C6H15NO....
Draw a Lewis structure, condensed structure and skeletal structure for a compound with molecular formula C6H15NO....
Draw the Lewis structure for SiH4. Draw the molecule by placing atoms on the grid and...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago