Question
In: Chemistry
Draw a Lewis structure for PO4 3
Draw a Lewis structure for PO4^3- that minimizes formal charges.
Solutions
Expert Solution
Well, actually, you can draw the Lewis diagram with single bonds, and all the oxygens have 3 lone pairs.
PO4^3- has 5+4x6+3 = 32 e-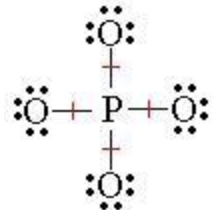
The only reason that you might want to include a double bond is because of overly strict adherence to formal charges. By making one of the bonds a double bond, you reduce the formal charge on P from +2 to +1 and one of the oxygens from -1 to 0.
The fact is that all four bonds are identical. If one of the bonds were a double bond, then one bond would have greater energy than the others. That is why you could use resonance to make all the bonds equivalent.
Related Solutions
For : TeCl4 ClF3 (PO4)3- please draw and list the lewis dot structure # of electron...
For
:
TeCl4
ClF3
(PO4)3-
please draw and list
the lewis dot structure
# of electron domains
the E.D. geometry
Molecular geometry drawing and angle
Polarity (yes or no)
Draw the Lewis structure for the I−3.
Draw the Lewis structure for the I−3.
Draw the Lewis structure for AsF3 Draw the Lewis dot structure. To change the symbol of...
Draw the Lewis structure for AsF3
Draw the Lewis dot structure. To change the symbol of an atom,
double-click on the atom and enter the letter of the new atom.
Draw the Lewis structure for CH3+. Be sure to show the formal
charge on any atom that has a non-zero formal charge.
Draw the Lewis dot structure.
Draw the Lewis structure for BrF3
Draw the Lewis dot structure.
draw the best Lewis structure for OCl2.
draw the best Lewis structure for OCl2.
draw the lewis structure for CH3CHBrCHBrCH2CH3 and for CH3CHFCH2OH
draw the lewis structure for CH3CHBrCHBrCH2CH3 and for
CH3CHFCH2OH
Draw the Lewis Structures and name all the isomers of C4H9Cl. Name Lewis Structure Draw the...
Draw the Lewis Structures and name all the isomers of
C4H9Cl.
Name
Lewis Structure
Draw the Lewis Structures and name all the isomers
of C2H2Cl2.
Name
Lewis Structure
Cycloalkanes
Would you predict that cyclopropane and cyclobutane are
highly stable molecules?
draw the Lewis dot structure for MgSe lewis dot structure for K2S as well please
draw the Lewis dot structure for MgSe
lewis dot structure for K2S as well please
Please draw lewis structure for SnBr2For the lewis structure of SnBr2, would you put a...
Please draw lewis structure for SnBr2For the lewis structure of SnBr2, would you put a
double bond between one of the Br's? Why or why not? Someone told
me you don't but I don't understand-- wouldn't you want to satisfy
the octet rule?
1. Draw a 3-D Lewis structure of the following and what is the formal charge of...
1. Draw a 3-D Lewis structure of the following and what is the
formal charge of the central atom;
a. I3+
b. NF3
c. POBr3
draw lewis structure for: BCl3 BCl2H BClH2
draw lewis structure for:
BCl3
BCl2H
BClH2
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
ADVERTISEMENT
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago