Question
In: Chemistry
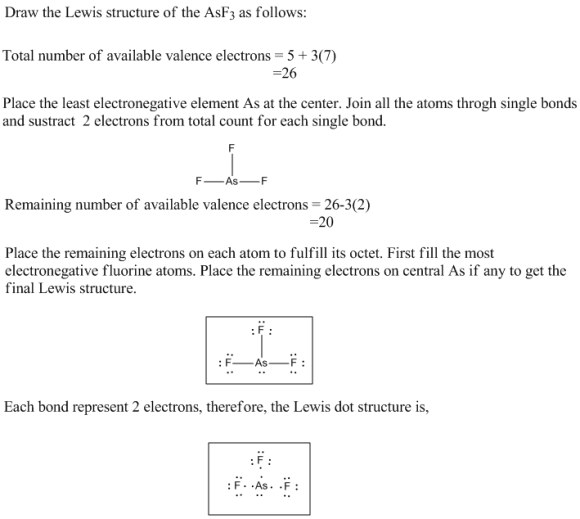
Draw the Lewis structure for AsF3 Draw the Lewis dot structure. To change the symbol of...
Draw the Lewis structure for AsF3
Draw the Lewis dot structure. To change the symbol of an atom,
double-click on the atom and enter the letter of the new atom.
Draw the Lewis structure for CH3+. Be sure to show the formal
charge on any atom that has a non-zero formal charge.
Draw the Lewis dot structure.
Draw the Lewis structure for BrF3
Draw the Lewis dot structure.
Solutions
Related Solutions
draw the Lewis dot structure for MgSe lewis dot structure for K2S as well please
draw the Lewis dot structure for MgSe
lewis dot structure for K2S as well please
Draw Lewis dot structure for Cl2CNH PI3 SCl2 I2
Draw Lewis dot structure for
Cl2CNH
PI3
SCl2
I2
Draw the Lewis Dot Structure for the following compounds and then determine a) the hybridization of...
Draw the Lewis Dot Structure for the following compounds and
then determine
a) the hybridization of the central atom
b) the electron group arrangement
c) the bond angle around the central atom
d) the molecular geometry.
a)
H3O+
b)
NO3-
c) O3
`
d)
HCN
e) CH3CH3
Draw Lewis dot structure (CH3)4 NCL A SALT
Draw Lewis dot structure (CH3)4 NCL A SALT
Consider SO3 a. Draw the Lewis dot structure that minimizes the formal charges and expands the...
Consider SO3
a. Draw the Lewis dot structure that minimizes the formal
charges and expands the octet.
b. Draw the Lewis dot structure that have resonance and do not
expand the octet.
Draw the Lewis dot structure of the following species and identify the number of pi bonds...
Draw the Lewis dot structure of the following species and
identify the number of pi bonds in each:
CS2
CH3Cl
NO2-
SO2
Questions: For each of the following molecules, draw the Lewis Dot Structure, identify the electronic and...
Questions:
For each of the following molecules, draw the Lewis Dot
Structure, identify the electronic and molecular geometry names,
and predict the bond angle of the bold atom. If you believe that
resonance structures exist, you may merely state so.
CH3I
SO3
CH3COOCH3
PCl3
ZnCl2
ZnCl42-
COHC6H4COOH
NH4+
ICl4-
RnF2
HArF
B2H6 (yes, this is a real molecule)
For what electronic geometries is the “octet rule” always
violated?
Though the octet rule is, indeed, frequently broken, it is never
broken...
For : TeCl4 ClF3 (PO4)3- please draw and list the lewis dot structure # of electron...
For
:
TeCl4
ClF3
(PO4)3-
please draw and list
the lewis dot structure
# of electron domains
the E.D. geometry
Molecular geometry drawing and angle
Polarity (yes or no)
Draw the lewis dot structure and all resonance structures for the dithiorcarbonate molecule (S2CO -2). Which...
Draw the lewis dot structure and all resonance structures for
the dithiorcarbonate molecule (S2CO -2).
Which lewis structure is the best for this structure?(formal
charge)
Acetylene, C2H2 and N2F2 have similar formulas. Draw the Lewis dot structure for each and identify...
Acetylene, C2H2 and N2F2 have similar formulas. Draw the
Lewis dot structure for each and identify the hybrid orbitals used
by C and N respectively. Also determine the type , σ and/or π, and
number of bonding used for each molecule.
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
ADVERTISEMENT


 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago