Question
In: Mechanical Engineering
Steam is compressed to a saturated vapor state at constant pressure. The steam started at 2...
Steam is compressed to a saturated vapor state at constant pressure. The steam started at 2 MPa and 365 C. What work was required and what was the specific heat transfer?
Please show work so I can actually learn!
Solutions
Expert Solution
We will first refer to the steam tables for the saturation properties of steam at Pressure of 2 MPa:
| Pressure (MPa) | Temp (C) | ug (kJ/kg) | vg (m3/kg) |
| 2 | 212.4 | 777.784 | 0.09962 |
Work done in compressing the superheated steam from superheated state to saturated vapor state:

where
- P = pressure = 2 MPa = 2000 kPa
- v1 = initial specific volume =
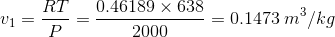
- R = characteristic gas constant =
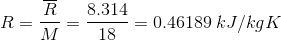
 = universal gas constant = 8.314 kg/kmolK
= universal gas constant = 8.314 kg/kmolK- M = molecular mass of water = 18 kg/kmol
- P = 2000 kPa
- T = initial temp = 365 C = 638 K
- R = characteristic gas constant =
- v2 = final specific volume = vg at 2 MPa = 0.09962 m3/kg (from the table above)


Negative sign indicates that work is being done on the system by the surroundings (compression work).
Now, change in internal energy = 
where
- u2 = final internal energy = ug at 2 MPa = 777.784 kJ/kg (from the table above)
- u1 = C x T1 = 1.667 x 638 = 1063.546 kJ/kg
- C = specific heat at constant volume at state 1 = 1.667 kJ/kgK (from the steam table)
- T1 = 365 C = 638 K


From the first law of thermodynamics, the specific heat transfer
= 

Negative heat interaction shows that heat is transferred from the system to the surroundings.
-----------------------------------------------
Kindly upvote if you are satisfied with my answer. :)
Related Solutions
A 10 kg steam initially at 2 MPa saturated vapor is inside an insulated piston cylinder....
120 kilograms of saturated water at 2.32 MPa pressure is heated to saturated vapor at same...
Saturated steam at a gauge pressure of 0.50 bar is to be used to heat a...
In a process plant, a large amount of saturated steam is available at a pressure of...
Saturated water vapor at 1000 kPa is throttled to 100 kPa. The velocity of the steam...
Refrigerant 22 undergoes a constant-pressure process within a piston–cylinder assembly from saturated vapor at 5.0 bar...
R22 is compressed adiabatically and reversibly from saturated vapor at -20 deg F to 200 psia....
A mass of 5kg of saturated water vapor at 150 kpa is heated at a constant...
Consider steam in an ideal Rankine cycle. The saturated vapor enters the turbine at 8.0 MPa.
An air stream is saturated with a vapor (B) at 130F and 1 atm pressure. It...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
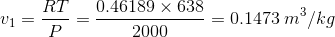
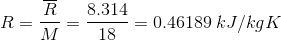
 = universal gas constant = 8.314 kg/kmolK
= universal gas constant = 8.314 kg/kmolK samet mamat answered 3 years ago
samet mamat answered 3 years ago