Question
In: Chemistry
The Lewis structure for the chlorate ion is Calculate the formal charge on the chlorine (Cl) atom
A) The Lewis structure for the chlorate ion is
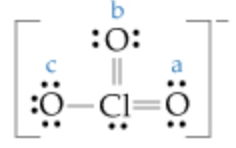
Calculate the formal charge on the chlorine (Cl) atom.
B)
Calculate the formal charge on each of the oxygen (O) atoms labeled \(\mathrm{a}, \mathrm{b},\) and \(\mathrm{c}\) in the following Lewis structure.
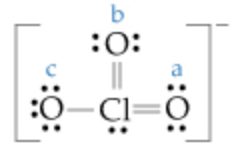
Express your answers as integers separated by commas.
C)
What are the formal charges on the sulfur (S), carbon (C), and nitrogen (N) atoms, respectively, in the resonance structure that contributes most to the stability of the thiocyanate ion, SCN\%u2212?
The possible resonance structures for the thiocyanate ion, SCN\%u2212, are
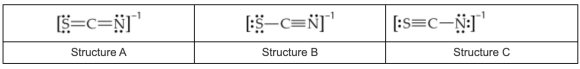
Express your answers as integers separated by commas.
Solutions
Expert Solution
Concepts and reason
A Lewis structure is a diagram that represents the chemical bonding between atoms of molecules and a lone pair of electrons that may exist in the molecule. It is also called a dot structure representing a lone pair and bond pair of electrons in the molecule. Lewis structure is used to draw covalently bonded molecules as well as coordination compounds.
Fundamentals
- Lewis's structure represents the bonding and lone pair of electrons in the molecule.
- Valence electrons are the electrons present in the outermost shell of an atom. Dots represent the electron position around the atoms, and lines or dot pairs are used to represent covalent bonds between atoms.
- The Lewis structure is based on the concept of the octet rule. So, the electrons shared in each atom should have 8 electrons in its outer shell.
- The formal charge (F.C) of the atom can be calculated by using the following formula.
$$ \left(\begin{array}{l} \text { Formal } \\ \text { charge (F.C) } \end{array}\right)=\left(\begin{array}{l} \text { no.ofvalence } \\ \text { electronsinatom } \end{array}\right)-\frac{1}{2}\left[\begin{array}{l} \text { no.ofbonding } \\ \text { electrons } \end{array}\right] -\left[\begin{array}{l}\text { no.ofnon }-\text { bonding } \\ \text { electrons }\end{array}\right] $$
Part A
The formal charge on the chlorine atom in the chlorate ion is zero.
Number of valance electron for Clis7
Number of bonding electrons for Clis10
Number of non - Bonding electrons for Clis2
Substituting these numbers in formal charge (FC)
\(\mathrm{FC}=7-\left(\frac{1}{2} \times 10\right)-2\)
\(=0\)
Therefore, the formal charge on the chlorine atom is zero in the chlorate ion.
Part B
Formal charge on oxygen \((\mathrm{a})=0\)
Formal charge on oxygen \((\mathrm{b})=0\)
Formal charge on oxygen \((\mathrm{c})=-1\)
Part B The charge on each oxygen atom is \(0,0,-1 .\)
Oxygen(a) :
Number of valance electron for \(\mathrm{O}(\mathrm{a})=6\)
Number of bonding electrons for \(\mathrm{O}(\mathrm{a})=4\)
Number of non \(-\) Bonding electrons for \(\mathrm{O}(\mathrm{a})=4\)
Substituting these numbers in formal charge :
\(\mathrm{FC}=6-\left(\frac{1}{2} \times 4\right)-4\)
\(=0\)
Oxygen(b) :
Number of valance electron for \(\mathrm{O}(\mathrm{b})=6\)
Number of bonding electrons for \(\mathrm{O}(\mathrm{b})=4\)
Number of non \(-\) Bonding electrons for \(\mathrm{O}(\mathrm{b})=4\)
Substituting these numbers in formal charge :
\(\mathrm{FC}=6-\left(\frac{1}{2} \times 4\right)-4\)
\(=0\)
Oxygen(c)
Number of valance electron for \(\mathrm{O}(\mathrm{c})=6\)
Number of bonding electrons for \(\mathrm{O}(\mathrm{c})=2\)
Number of non \(-\) Bonding electrons for \(\mathrm{O}(\mathrm{c})=6\)
Substituting these numbers in formal charge :
\(\mathrm{FC}=6-\left(\frac{1}{2} \times 2\right)-6\)
\(=-1\)
Therefore, the formal charge on each oxygen atom is \(0,0,-1 .\)
Part C
The most stable structure is structure \(B\) :
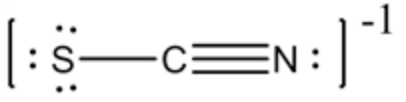
For the structure B:
Formal charge on sulfur(S) \(=0\)
Formal charge on carbon \((\mathrm{C})=0\)
Formal charge on nitrogen \((\mathrm{N})=-1\)
Part C The formal charge on sulfur, carbon and nitrogen is 0,0,-1 .
Sulfur(S) :
Number of valance electron for \(\mathrm{S}=6\)
Number of bonding electrons for \(\mathrm{S}=4\)
Number of non - Bonding electrons for \(\mathrm{S}=4\)
Substituting these numbers in formal charge :
\(\mathrm{FC}=6-\left(\frac{1}{2} \times 4\right)-4\)
\(=0\)
\(\operatorname{Carbon}(\mathrm{C})\)
Number of valance electron for \(\mathrm{C}=4\)
Number of bonding electrons for \(\mathrm{C}=8\)
Number of non \(-\) Bonding electrons for \(\mathrm{C}=0\)
Substituting these numbers in formal charge :
\(\mathrm{FC}=4-\left(\frac{1}{2} \times 8\right)-0\)
\(=0\)
Nitrogen \((\mathrm{N}):\)
Number of valance electron for \(\mathrm{N}=5\)
Number of bonding electrons for \(\mathrm{N}=4\)
Number of non \(-\) Bonding electrons for \(\mathrm{N}=4\)
Substituting these numbers in formal charge :
\(\mathrm{FC}=5-\left(\frac{1}{2} \times 4\right)-4\)
\(=-1\)
Therefore, the formal charge on sulfur, carbon and nitrogen is 0,0,-1
Related Solutions
Write the Lewis structure. Calculate the formal charge on the center atom. Show resonance if it...
In the Lewis structure for BeCl2 , what is the formal charge on the Be atom?...
What is the formal charge on the C atom in the correct or best Lewis structure...
Draw the Lewis structure for CH2MgBr and give the formal charge of each atom
c2h5+ lewis structure and formal charge
draw the best lewis structure for SF42- and calculate the formal charge on sulfur
NOCl (N is the central atom) Draw the lewis structure Number of Valence electrons Formal charge
what is the lewis dot structure and formal charge for CH2O?
SCl2 Draw the lewis structure Number of Valence electrons Formal charge
17.56: Chlorine Dioxide (ClO2) is produced by the following reaction of chlorate (ClO3-) with Cl- in...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...
- How many grays is this?Part A A dose of 4.7 Sv of γ rays in a...
- how to operate a business?
- Discuss pros and cons of using twins to estimate the rate of return to school
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago