Question
In: Chemistry
They hydrogen atom is not actually electronegative enough to form bonds to xenon.
They hydrogen atom is not actually electronegative enough to form bonds to xenon. Were the xenon-hydrogen bond to exist, what would be the structure of XeH4?
Double-click any atom and type Xe to change the label.
Draw the molecule by placing atoms on the grid and connecting them with bonds. Show all lone pairs of electrons.
Solutions
Expert Solution
Concepts and reason
The attraction of atoms or ions forms the chemical bond. Due to electronegativity, atoms can attract electrons in a chemical bond. A chemical bond is also formed with the help of valance electrons in an atom. Additionally, the bond should obey the octet rule.
Fundamentals
When the bond formed from elements has a higher electronegativity difference, it tends to have ionic bonding. Example: HF In hydrogen fluoride, hydrogen is less electronegative, and fluorine is more electronegative. The higher electronegativity difference leads to ionic bonding. The bonds formed from the elements have more or less electronegativity difference; they tend to have covalent bonding. Example: \(\mathrm{CH}_{4}\) In methane, carbon contains 4 valance electrons, and 4 hydrogen atoms contain 1 electron each. Therefore, both carbon and hydrogen share their electrons and form a covalent bond. The electronegativity difference between carbon and 4 hydrogen atoms is less.
\(\mathrm{Xe}=8 \)
\(4(\mathrm{H})=4\)
Total \(=12\)
The electronic configuration of \(\mathrm{Xe}\) is \([\mathrm{Kr}] 4 \mathrm{~d}^{10} 5 \mathrm{~s}^{2} 5 \mathrm{p}^{6} .\) For \(\mathrm{Xe},\) there are 8 electrons present in the outermost shell. Therefore, the valance electron in \(\mathrm{Xe}\) is 8 . Hydrogen has 1 valence electron. Therefore, 4 hydrogen atoms have 4 valance electrons. The valance electron of xenon is 8 and the valance electron of 4 hydrogen atoms is \(4 .\) Therefore, there are totally 12 valance electrons in \(\mathrm{XeH}_{4}\)
The structure of \(\mathrm{XeH}_{4}\)
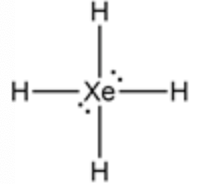
Taking xenon as the central atom, 4 hydrogen atoms form a bond. From the 12 valance electrons, 8 electrons form a bond. The remaining four electrons are placed as a lone pair of electrons around the central atom and form an octahedral complex.
Related Solutions
They hydrogen atom is not actually electronegative enough to form bonds to xenon. Were the xenon-hydrogen...
The hydrogen atom is not actually electronegative enough to form bonds to xenon. Were the xenon-hydrogen...
1. when an electronegative atom shares electrons with a less electronegative atom, what is the result?...
Can the carbon atom in a carbonyl group form hydrogen bonds with water molecules? Can the...
Water molecules do NOT:a. Form hydrogen bonds with other polar substancesb. Form transient hydrogen...
Which of the following molecules can form hydrogen bonds?
An electron collides elastically with a stationary hydrogen atom. The mass of the hydrogen atom is...
hydrogen bonds... a) are the strongest but can be weak b) form between two hydrogen atoms...
Answer the following questions using the Bohr model of the hydrogen atom. a) A hydrogen atom...
How many hydrogen bonds can AMP form with water?
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago