Question
In: Chemistry
Assign formal charges to each atom in the O3 molecule shown here
Assign formal charges to each atom in the O3 molecule shown here. Be sure to click the + or - button before clicking on the molecule.

Solutions
Expert Solution
Concepts and reason
The concept used to solve this problem is the formal charge calculation of the oxygen atoms present in the ozone molecule.
Fundamentals
A charge that is assigned to an atom, which is present in the molecule, is represented as a formal charge. In two bonded atoms, a formal charge assumes that the electrons are equally shared between them. The formal charge is calculated using the following formula:
Formal charge \(=\mathrm{V}-\mathrm{N}-\frac{\mathrm{B}}{2}\)
Here, \(\mathrm{V}\) is the number of valence electrons, \(\mathrm{N}\) is the number of non-bonding electrons and \(\mathrm{B}\) is the number of bonding electrons.
The structure is as follows:
Calculate the formal charge for the circled oxygen in ozone molecule as follows:
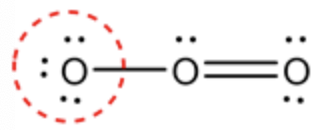
Formal charge \(=\mathrm{V}-\mathrm{N}-\frac{\mathrm{B}}{2}\)
\(\begin{aligned}=& 6-6-\frac{2}{2} \\ &=-1 \end{aligned}\)
Explanation | Common mistakes | Hint for next step
The marked oxygen atom has 6 valence electrons. It contains 6 non-bonding electrons. The marked oxygen atom is bonded with one oxygen atom. Therefore, the bonding electron of oxygen is \(2 .\) Substitute the values in a formal charge formula to determine the formal charge of oxygen (marked).
The structure is as follows:
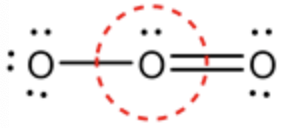
Calculate the formal charge for the circled oxygen in ozone molecule as follows:
Formal charge \(=\mathrm{V}-\mathrm{N}-\frac{\mathrm{B}}{2}\)
\(=6-2-\frac{6}{2}\)
\(=+1\)
Explanation | Common mistakes | Hint for next step
The oxygen atom in the middle has 6 valence electrons and has 2 non-bonding electrons. The central oxygen atom is bonded with 1 double bond and 1 single bond. So, there is a total of 3 bonds containing 6 electrons (bonding electrons). The formal charge of the middle oxygen atom can be determined by substituting the values in a formal charge formula.
The structure is as follows:
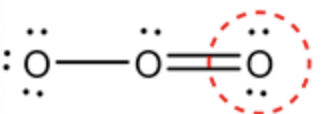
Calculate the formal charge for the circled oxygen in ozone molecule as follows:
Formal charge \(=\mathrm{V}-\mathrm{N}-\frac{\mathrm{B}}{2}\)
\(=6-4-\frac{4}{2}\)
\(=0\)
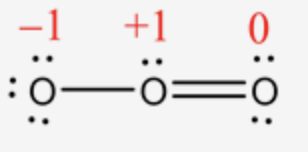
The formal charge of each oxygen atom in \(\mathrm{O}_{3}\) molecule are labeled as follows:
The oxygen atom present on the right side contains 6 valence electrons which have 2 non-bonded electrons. The marked oxygen is bonded with 1 double bond with a central oxygen atom. So, the number of bonding electrons is \(4 .\) The formal charge of the corner oxygen atom (right corner) can be determined by substituting the values in the formal charge formula.
The formal charge of each oxygen atom in \(\mathrm{O}_{3}\) molecule are labeled as follows:

Related Solutions
Draw 3 resonance structures for NOCl and assign formal charges to each atom. State which resonance...
Write Lewis structure that obeys the octet rule for BrO−3 and assign formal charges to each...
Draw the Lewis Structure for each molecule. Remember that the best structure will have a few Formal Charges as possible
For each of the following: • Assign Oxidation States (Numbers) to each atom in reactants and...
Determine the formal charge of each atom in the reactants and the products. Reactants:
add formal charges to each resonance form of HCNO below
Which is FALSE about formal charges and resonance forms? 1. The sum of the formal charges...
Assign oxidation states to each atom in each of the following species. Part A N2 Part...
Draw the Lewis structure for CH2MgBr and give the formal charge of each atom
. A double-stranded DNA molecule with the sequence shown here produces, in vivo, a polypeptide that...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago