Question
In: Chemistry
1.567 g of an unknown Mohr’s salt was dissolved in 80 mL of the sulfuric acid...
1.567 g of an unknown Mohr’s salt was dissolved in 80 mL of the sulfuric acid / phosphoric acid mixture solution. This iron solution was then titrated with a potassium permanganate solution (0.02M), what will be the volume at the equivalence point for the titration? Show all your calculations including formulas.
Solutions
Expert Solution
The balanced equation is :
2KMnO4 + 8H2SO4 + 10
FeSO4.(NH4)2SO4.6H2O
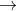 K2SO4 + 2MnSO4 +
5Fe2(SO4)3 + 10
(NH4)2SO4 + 48H2O
K2SO4 + 2MnSO4 +
5Fe2(SO4)3 + 10
(NH4)2SO4 + 48H2O
Molar mass of Mohr's salt is 284 g/mol
Number of moles of Mohr's salt , n = mass / molar mass
= 1.567 g / 284(g/mol)
= 5.52x10-3 mol
According to the balanced chemical equation ,
10 moles of Mohr's salt reacts with 2 moles of KMnO4
5.52x10-3 mol of Mohr's salt reacts with M moles of KMnO4
M = (2x5.52x10-3 ) / 10
= 1.10 x10-3 mol
Volume of KMnO4 at Equilvalence point , V = number of moles / Molarity
= (1.10 x10-3 mol) / 0.02 M
= 0.055 L
= 0.0552 x103 mL Since 1 L = 10 3 mL
= 55.2 mL
Related Solutions
Suppose that 0.323 g of an unknown sulfate salt is dissolved in 50 mL of water....
A 0.1276 g sample of an unknown monoprotic acid was dissolved in 25.0 mL of water...
A CHEM 2242 student dissolved 69.322 g of an unknown monoprotic acid in a 250 mL...
Pure (100%) sulfuric acid has a density of 1.84 g/ml. How many mL of sulfuric acid...
A 1.31 gram sample of an unknown monoprotic acid is dissolved in 50.0 mL of water...
A sample of unknown acid is dissolved into 100-mL of water (assume negligible volume change) and...
A 6.31 g sample of benzoic acid was dissolved in water to give 56.5 mL of...
20.26 grams of unknown 1 (with molar mass 203.67 g/mol) was dissolved in 182.7 mL of...
You have a 1.153 g sample of an unknown solid acid, HA, dissolved in enough water...
A recrystallization of 3.00 g of benzoic acid is to be performed using 80 ml of...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago