Question
In: Biology
Describe why Histidine is often found in active sites and is involved in the catalytic mechanisms...
Describe why Histidine is often found in active sites and is involved in the catalytic mechanisms of enzymes. What role does it generally play in catalytic mechanisms? Draw an example of a catalytic mechanism that involves Histidine as a critical amino acid residue.
Solutions
Expert Solution
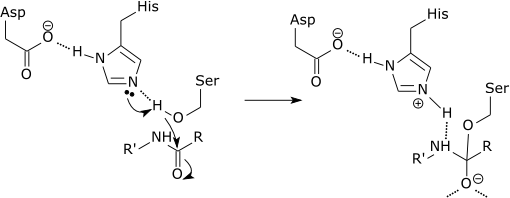
Histidine has an imidazole ring which can be protonated and deprotonated depending on the physiological pH. So, it can perform different roles in catalysis. Its pKa is 6 so it can be protonated and deprotonated very easily at physiological pH. When protonated it acts as a proton donor (acid) and when it is deprotonated it acts as a proton acceptor (base). Histidine also acts as electron donor (lewis base) to act as ligand for a metal ion for the metalloenzymes.
Reaction involving histidine: Protons are transferred from serine to histidine. Here histidine acts as a base.
Related Solutions
Histidine often shows up as a key player in enzymatic reaction mechanisms and likewise often serves...
Describe the active (binding) sites of Hb, Hc and Hr.
Describe the possible mechanisms involved in the development of atherosclerosis. Describe mechanisms of development of atheroschlerosis....
Describe two specific catalytic mechanisms that enzymes may use to speed up a reaction and describe...
Describe a several types of defensive mechanisms ( both passive types and active types) that prey...
In your own words, describe three mechanisms of transcriptional regulation, that are involved in controlling the...
Explain the different forms of membrane transport: Passive, Facilitated, Active. Describe the bulk transport mechanisms of...
8. Describe an electrospray ionization source and the mechanisms involved for the generation of gas-phase ions....
Investment Bankers often become involved with the mergers and acquisitions of firms. So, why might a...
1.Describe the various mechanisms involved in the regulation gene transcription. •RNAi •Epigenetics •Regulatory elements can u...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
 gladiator answered 3 years ago
gladiator answered 3 years ago