Question
In: Chemistry
The following sets of quantum numbers, listed in the order and were written for the last electrons added to an atom
The following sets of quantum numbers, listed in the order n, l, ml, and ms were written for the last electrons added to an atom. Identify which sets are valid and classify the others by the rule or principle that is violated. Drag each item to the appropriate bin.
Quantum Number order: n, l, ml, ms

Solutions
Expert Solution
Concept and reason
The concept used is to identify the valid set of quantum numbers for the last electrons added to an atom.
Fundamentals
The four quantum numbers required to describe an atom are principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number. Principal quantum number: It describes the energy level of an electron. It is denoted by n. The value of \(\mathrm{n}\) ranges from 1 to the level of the electron in the outermost orbit. Azimuthal quantum number: It describes the sub-shell of an electron. I denote it.
The value of I ranges from 0 to \((n-1)\)
Magnetic quantum number: It describes the energy levels of the sub-shell of an electron. It is denoted by \(m_{l}\).
The value of \({ }^{m_{l}}\) ranges from \(-l\) to \(+l\).
Spin quantum number: It describes the spin of an electron. It is denoted by \(m_{s}\). The value of \({m_{s}}\) can either be \(+1 / 2\) or \(-1 / 2\) According to Pauli's exclusion principle, no two electrons can have same four quantum numbers.
Consider the set as follows:
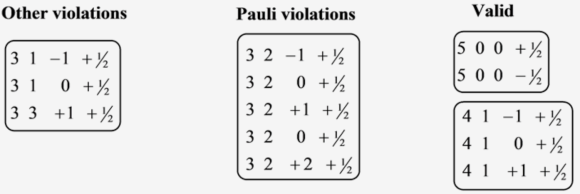
\(\begin{array}{lll}5 & 0 & 0 & +1 / 2\end{array}\)
\(\begin{array}{llll}5 & 0 & 0 & -1 / 2\end{array}\)
It is a valid set.
Explanation
Here, the value of \(n\) is \(5 .\) So, \(l\) can be 0,1,2,3,4 .
Here, \(l=1 .\) So, \(m_{l}\) can be -1,0,+1
Here, \(m_{l}=0 .\) So, \(m_{s}\) can be \(+1 / 2,-1 / 2\) There is no repetition of the quantum numbers. [Hint for the next step] Identify whether the given set is valid or not.
Consider the set as follows:
\(\begin{array}{lll}4 & 1 & -1 & +1 / 2\end{array}\)
\(\begin{array}{lll}4 & 1 & 0 & +1 / 2\end{array}\)
\(41+1+1 / 2\)
It is a valid set.
Explanation
Here, the value of \(n\) is \(4 .\) So, \(l\) can be 0,1,2,3 .
Here, \(l=1 .\) So, \(m_{l}\) can be -1,0,+1
Here, \(m_{l}=-1,0,+1 .\) So, \(m_{s}\) can be \(+1 / 2,-1 / 2\) There is no repetition of the quantum numbers. [Hint for the next step] Identify whether the given set is valid or not.
Consider the set as follows:
\(\begin{array}{lll}3 & 2 & -1 & +1 / 2\end{array}\)
\(320+1 / 2\)
\(32+1+1 / 2\)
\(320+1 / 2\)
\(32+2+1 / 2\)
It is not a valid set. There is a violation of Pauli exclusion principle.
Explanation
Here, the value of \(n\) is \(3 .\) So, \(l\) can be 0,1,2 .
Here, \(l=2 .\) So, \(m_{l}\) can be -2,-1,0,+1,+2
Here, \(m_{l}=-2,-1,0,+1,+2 .\) So, \(m_{s}\) can be \(+1 / 2,-1 / 2\) There is repetition of the quantum numbers.
\(320+1 / 2\) is repeated twice. [Hint for the next step] Identify whether the given set is valid or not.
Consider the set as follows:
\(\begin{array}{lll}3 & 1 & -1 & +1 / 2\end{array}\)
\(\begin{array}{lll}3 & 1 & 0 & +1 / 2\end{array}\)
\(33+1+1 / 2\)
It is not a valid set. There are other violations.
The valid and non-valid sets of quantum numbers are as follows:
Here, the value of \(n\) is \(3 .\) So, \(l\) can be 0,1,2 .
Here, \(l=1 .\) So, \(m_{l}\) can be \(-1,0,+1 . \mid\) cannot be 3 .
Here, \(m_{l}=-1,0,+1 .\) So, \(m_{s}\) can be \(+1 / 2,-1 / 2\).
There is a value of \(l=3\) that is not possible for the given quantum number, \(n=3\).
Related Solutions
how many electrons in an atom could have these sets of quantum numbers?
How many electrons in an atom can have the following sets of quantum numbers? Enter the...
How many electrons in an atom could have these sets of quantum numbers? n=3
write the quantum numbers for the electrons in the highest energy level of the atom? then...
List the quantum numbers for the electrons in the ground state of a neon atom. What...
Write out the quantum numbers for the electrons in a Neon atom..and explain please.
Give the maximum number of electrons in an atom for the given quantum numbers or designations....
Which one of the following sets of quantum numbers could be those of the distinguishing (last)...
Which of the following sets of quantum numbers are not allowed? For the sets that are...
Two of the three electrons in a lithium atom have quantum numbers of n = 1,...
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago