Question
In: Chemistry
how many electrons in an atom could have these sets of quantum numbers?
How many electrons in an atom could have these sets of quantum numbers?
n=2 --> number electrons = ?
n=5, l=2 --> number electrons= ?
n=7, l=1, ml=-1 --> number electrons= ?
Solutions
Expert Solution
Concepts and reason
Quantum numbers describes the energy states and orbitals of the atom. Quantum numbers are classified in to four types. They are,
1.Principle quantum number
2.Angular momentum quantum number
3.Magnetic quantum number
4.Spin quantum number
Fundamentals
Principle quantum number is denoted with \(\mathrm{n}\) and it describes the energy level of the orbital. n values are started from \(1,2,3,\) so on. Calculate the number of electron in \(\mathrm{n}\) shell by using the formula \(2 \mathrm{n}^{2}\). Angular quantum number is denoted with I and its describes the shape of the orbital
If \(\mathrm{I}=0\) then sublevel is \(\mathbf{s}\)
If \(\mathrm{I}=1\) then sublevel is \(\mathbf{p}\)
If \(\mathrm{I}=2\) the sublevel is \(\mathbf{d}\)
If \(\mathrm{I}=3\) the sublevel is \(\mathbf{f}\)
Magnetic quantum number is denoted with I and it describes the orientation of the orbital in space. Number of orbitals possible for the given \(\mathrm{n}\) value is calculated by using \(\mathrm{n}^{2}\).
\(\mathrm{N}\) value is 2 . So, calculate the number of electron by using following formula \(2 \mathrm{n}^{2}\)
Number of electrons \(=2 \mathrm{n}^{2}\)
\(=2(2)^{2}\)
\(=8\) electrons
Thus, the number electrons present in \(\mathrm{n}=2\) shell are 8 electrons.
On substituting \(\mathrm{n}\) value in \(2 \mathrm{n}^{2}\) we get the number of electrons.
\(\mathrm{n}=5, l=2\)
\(l=2\) means \(\mathrm{d}\) sublevel and \(\mathrm{n}=5\) indicates 5 th energy level.
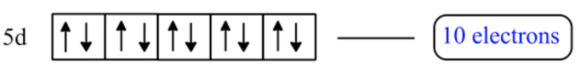
Thus, the number electrons present in \(\mathrm{n}=5\) and \(l=2\) are 10 electrons.
\(\mathrm{n}=5\) and \(l=2\) means \(5 \mathrm{~d}\) sublevel. In this level five orbitals are present. Each orbital can accommodate two electrons. So, number of electrons present in \(\mathrm{n}=5\) and \(l=2\) is 10 electrons.
\(\mathrm{n}=7, l=1, \mathrm{~m}_{l}=-1\)
\(l=1\) means \(\mathrm{p}\) sublevel and \(\mathrm{n}=7\) indicates 7 th energy level.
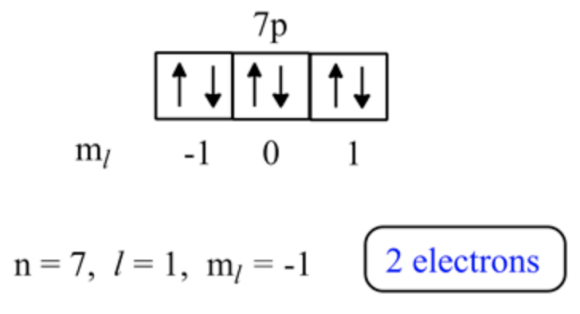
Thus, the number electrons present in \(\mathrm{n}=7, l=1,\) and \(\mathrm{m}_{l}=-1\) are 2 electrons.
\(\mathrm{n}=7, l=1,\) and \(\mathrm{m}_{l}=-1\) means \(7 \mathrm{p}\) sublevel. In this level three orbitals are present. Each orbital can accommodate two electrons. However, \(\mathrm{m}_{l}=-1\) contains 2 electrons.
Related Solutions
How many electrons in an atom could have these sets of quantum numbers? n=3
How many electrons in an atom can have the following sets of quantum numbers? Enter the...
How many ORBITALS in an atom could have these sets of quantum numbers? Please Explain.
How many orbitals in an atom could have these sets of quantum numbers? n=4,ℓ=3,mℓ=0
How many electrons could have the following set of quantum numbers? (a) n =4, ℓ =3...
The following sets of quantum numbers, listed in the order and were written for the last electrons added to an atom
How many electrons in an atom can have each of the following quantum number or sublevel...
How many orbitals in an atom could have these sets of quantumnumbers?How many orbitals...
Two of the three electrons in a lithium atom have quantum numbers of n = 1,...
write the quantum numbers for the electrons in the highest energy level of the atom? then...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago