Question
In: Physics
A phase transition from solid to liquid takes place at constant pressure and temperature. This is...
A phase transition from solid to liquid takes place at constant pressure and temperature. This is a closed PVT system. (a) Show that the Gibbs free energy G = U - TS + PV is a constant. (b) The solid and liquid phases are in equilibrium at a temperature T and pressure P, where G(solid) = G(solid)(T,P) and G(liquid) = G(liquid)(T,P). The phases are also in equilibrium at the neighboring temperature T +dT and pressure P + dP, where G(solid) = G(solid)(T+dT, P+dP) and G(liquid) = G(liquid)(T+dT, P+dP). Show that at temperature T, (dP/dT) = (S(liquid) - S(solid)) / (V(liquid) - V(solid)) where S and V denote entropy and volume respectively.
Solutions
Expert Solution
Free energy, in thermodynamics, energy-like property or state function of a system in thermodynamic equilibrium. Free energy has the dimensions of energy, and its value is determined by the state of the system and not by its history. Free energy is used to determine how systems change and how much work they can produce. It is expressed in two forms: the Helmholtz free energy F, sometimes called the work function, and the Gibbs free energy G. If U is the internal energy of a system, PV the pressure-volume product, and TS the temperature-entropy product (T being the temperature above absolute zero), then F = U − TS and G = U + PV − TS. The latter equation can also be written in the formG = H – TS, where H = U + PV is the enthalpy. Free energy is an extensive property, meaning that its magnitude depends on the amount of a substance in a given thermodynamic state.
The Gibbs free energy total differential natural variables may be derived via Legendre transforms of the internal energy.
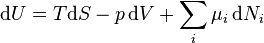 .
.
Because S, V, and Ni are extensive variables, Euler's homogeneous function theorem allows easy integration of dU
 .
.
The definition of G from above is
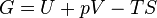 .
.
Taking the total differential, we have
 .
.
Replacing dU with the result from the first law gives
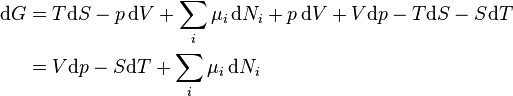 .
.
The natural variables of G are then p, T, and {Ni}.
Related Solutions
A gas phase reaction takes place in a syringe at a constant temperature and pressure. If...
A phase diagram is a temperature-pressure plot that summarizes the conditions under which a substance exists as a solid, liquid, or gas.
The liquid-phase reaction A---> B + C takes place in a series of two CSTRs...
Water changes between solid phase (as ice) and liquid phase at 0℃. and between liquid phase and gas phase (as water vapor) at 100 ℃.
When adding heat to a substance at its phase transition temperature, why does the temperature of...
In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines...
air at a temperature of 500c id compressed at a constant pressure of 1.2mpa from a...
A sample of water in the vapor phase (no liquid present) in a flask of constant...
1. How is the temperature dependence of the solubility of a solid in a liquid exploited...
Water is cooled to 32K, and it appears to be on the solid phase on the phase changing diagram as the temperature is low.
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 genius_generous answered 3 years ago
genius_generous answered 3 years ago