Question
In: Physics
A 2.00 mol sample of an ideal gas with a molar specific heat of CV =...
A 2.00 mol sample of an ideal gas with a molar specific heat of CV = 5/2 R always starts at pressure 1.50 ✕ 10^5 Pa and temperature 350 K. For each of the following processes, determine the final pressure (Pf, in kPa), the final volume (Vf, in L), the final temperature (Tf, in K), the change in internal energy of the gas (ΔEint, in J), the energy added to the gas by heat (Q, in J), and the work done on the gas (W, in J) a.) The gas is heated at constant pressure to 460 K. b.) The gas is heated at constant volume to 460 K. c.) The gas is compressed at constant temperature to 200 kPa d.) The gas is compressed adiabatically to 200 kPa.
Solutions
Related Solutions
A 2.00 mol sample of an ideal gas with a molar specific heat of CV =...
A 2.00 mol sample of an ideal gas with a molar specific heat of
CV =
5
2
R
always starts at pressure 1.00 ✕
105
Pa and temperature 350 K. For each of the following
processes, determine the final pressure (Pf, in kPa), the final volume
(Vf, in
L), the final temperature (Tf, in K), the change in
internal energy of the gas (ΔEint,
in J), the energy added to the gas by heat (Q, in J), and
the...
A 2.00 mol sample of an ideal gas with a molar specific heat of CV =...
A 2.00 mol sample of an ideal gas with a molar specific heat of
CV = (5/2)R always
starts at pressure 2.00 ✕ 105 Pa and temperature 300 K.
For each of the following processes, determine the final pressure
(Pf, in kPa), the final volume
(Vf, in L), the final temperature
(Tf, in K), the change in internal
energy of the gas (ΔEint, in J), the energy
added to the gas by heat (Q, in J), and the work done...
A 1.50 mol sample of an ideal gas with a molar specific heat of CV =...
A 1.50 mol sample of an ideal gas with a molar specific heat of
CV = 5/2 R always
starts at pressure 2.00 ✕ 105 Pa and temperature 250 K.
For each of the following processes, determine the final pressure
(Pf, in kPa), the final volume
(Vf, in L), the final temperature
(Tf, in K), the change in internal
energy of the gas (ΔEint, in J), the energy
added to the gas by heat (Q, in J), and the work...
A 2.50 mol sample of an ideal gas with a molar specific heat of CV =...
A 2.50 mol sample of an ideal gas with a molar specific heat of
CV = 5/2 R always starts at pressure 1.50 • 10^5 Pa and temperature
300 K. For each of the following processes, determine the final
pressure (Pf, in kPa), the final volume (Vf, in L), the final
temperature (Tf, in K), the change in internal energy of the gas
(ΔEint, in J), the energy added to the gas by heat (Q, in J), and
the work...
An ideal gas has a constant volume specific heat cv as a function of temperature. Find...
An ideal gas has a constant volume specific heat cv as a
function of temperature. Find the
change in internal energy and enthalpy if the gas is heated from a
temperature of 300K to 600K.
cv(T) = 716.66 + 0.4T + 0.000667T2 J/kg.K
Also, sketch the constant pressure specific heat as a function
of temperature and mention the
point T = 400K on the cp – T diagram. Assume that the gas constant
of the given ideal gas is
286.9...
A specific type of ideal gas has a specific heat capacity at constant pressure (cp=cv+R) that...
A specific type of ideal gas has a specific heat capacity at
constant pressure (cp=cv+R) that is a
function of temperature T, such that cp=0.48T+885, where
cp has units of J/kg/K and T has units of K. The gas,
which is initially at T1 = 314 K and P1 = 1x105 Pa,
undergoes a reversible adiabatic process such that its final
temperature is T2 = 772 K. Calculate the pressure of the gas (in
Pa) in this final state. Assume...
Consider a sample containing 2.00 mol of an ideal diatomic gas. Assuming the molecules rotate but...
Consider a sample containing 2.00 mol of an ideal diatomic gas.
Assuming the molecules rotate but do not vibrate, find a) the total
heat capacity of the sample at constant volume and b) the total
heat capacity at constant pressure. C and d) repeat parts a) and b)
assuming the molecules both rotate and vibrate. EXPLAIN how you got
these answers.
Answers should be:
a: 2mol*5/2 R
b: 2 mol*7/2 R
c: 2 mol * 7/2 R
d: 2 mol*9/2...
An extremely small sample of an ideal diatomic gas (with a molar mass of 28 g/mol)...
An extremely small sample of an ideal diatomic gas (with a molar
mass of 28 g/mol) has the following distribution of molecular
speeds: 1 molecule moving at 100 m/s, 2 molecules at 200 m/s, 4 at
300 m/s, and 3 at 400 m/s.
What is the rms speed of the distribution? What is the average
kinetic energy of translational motion per molecule? What is the
temperature of this sample?
4.1) A perfect gas has a constant volume molar heat capacity of CV ,m 1.5...
4.1) A perfect gas has a constant volume molar heat capacity of
CV ,m 1.5 R and a constant pressuremolarheatcapacityofCp,m
2.5R.Fortheprocessofheating2.80molofthisgaswitha 120 W heater for
65 seconds, calculate a) q, w, T, and U for heating at a constant
volume, b) q, w, T, and H for heating at a constant pressure.
4.2) Determine the heat capacity Cp and the molar heat
capacity Cp,m of a solid sample from the observation that
transferring the sample with n =...
The temperature of 2.00 mol of an ideal monatomic gas is raised 15.0 K at constant...
The temperature of 2.00 mol of an ideal monatomic gas is raised
15.0 K at constant volume. What are (a) the work W done by the gas,
(b) the energy transferred as heat Q , (c) the change
?Eint in the internal energy of the gas, and (d) the
change ?K in the average kinetic energy per atom
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
ADVERTISEMENT

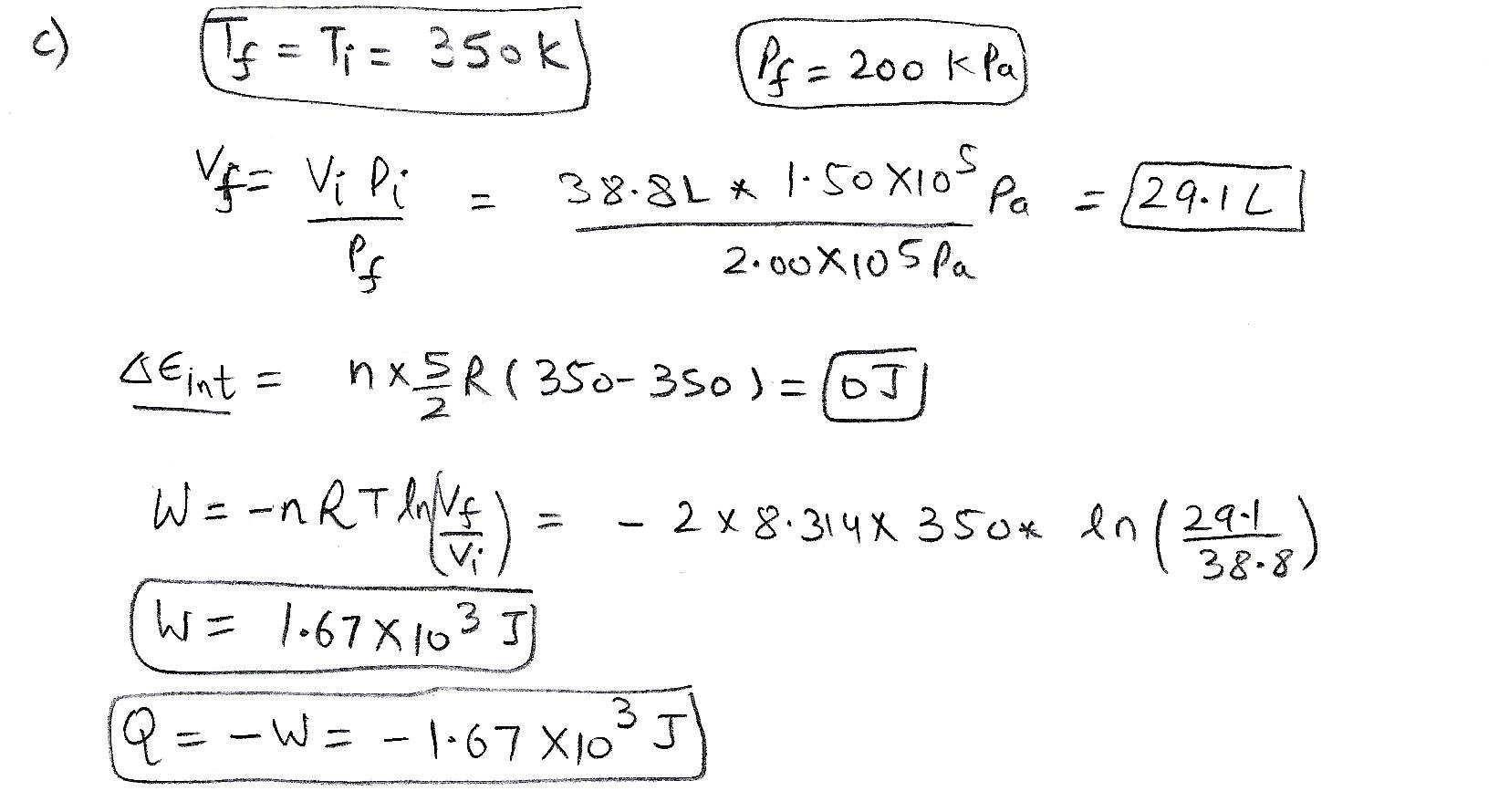
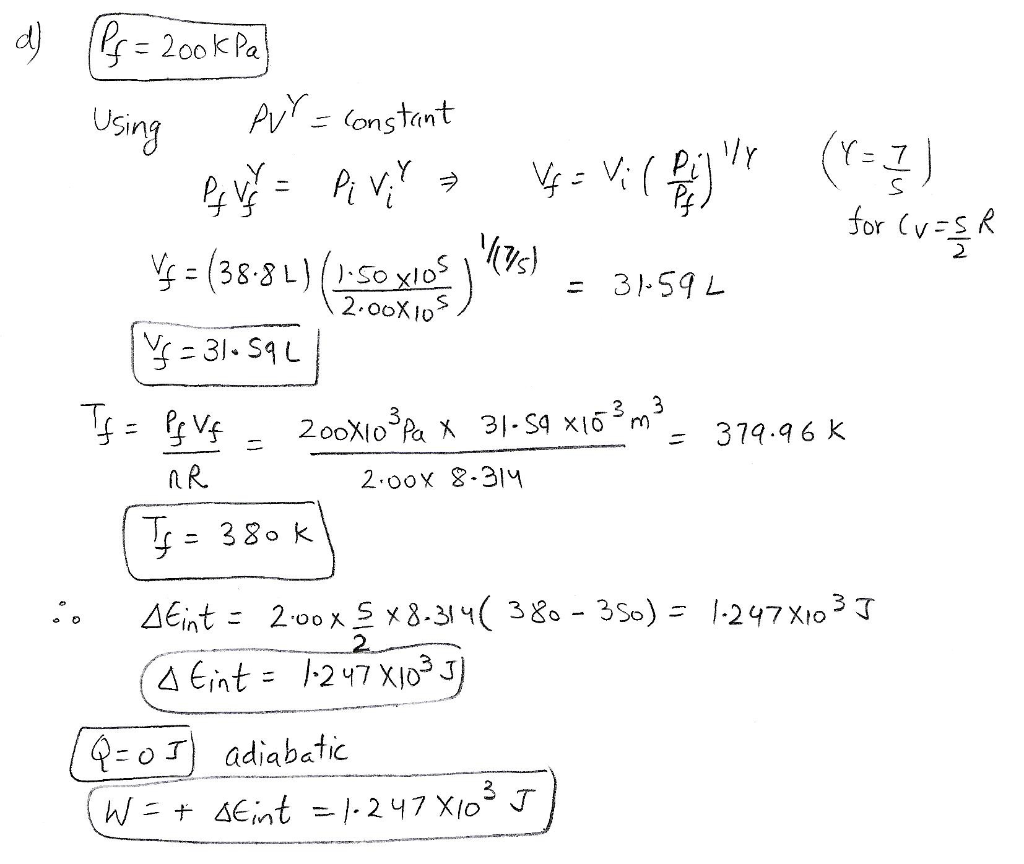
 genius_generous answered 1 month ago
genius_generous answered 1 month ago