Question
In: Chemistry
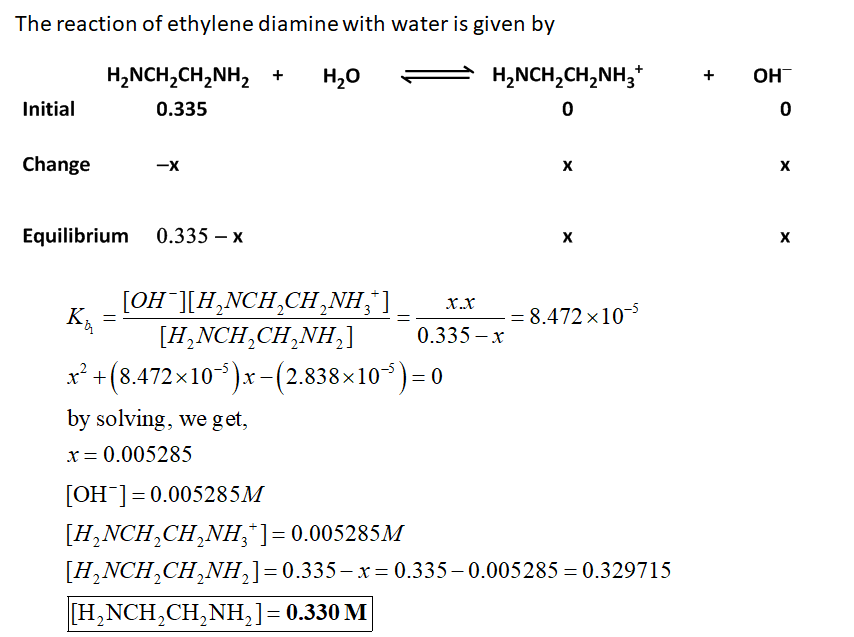
Calculate the pH of a 0.335 M solution of ethylenediamine (H2NCH2CH2NH2). The pKa values for the...
Calculate the pH of a 0.335 M solution of ethylenediamine (H2NCH2CH2NH2). The pKa values for the acidic form of ethylenediamine ( H3NCH2CH2NH3 ) are 6.848 (pKa1) and 9.928 (pKa2).
pH=
Calculate the concentration of each form of ethylenediamine in this solution at equilibrium.
[H2NCH2CH2NH2]=
[H2NCH2CH2NH3+]=
[H3+NCH2CH2NH3+]=
Solutions
Related Solutions
Calculate the pH of a 0.228 M solution of ethylenediamine (H2NCH2CH2NH2). The pKa values for the...
Calculate the pH of a 0.228 M solution of ethylenediamine
(H2NCH2CH2NH2). The pKa values for the acidic form of
ethylenediamine ( H3NCH2CH2NH3 ) are 6.848 (pKa1) and 9.928 (pKa2).
AND Calculate the concentration of each form of ethylenediamine in
this solution at equilibrium.
Calculate the pH of a 0.368 M solution of ethylenediamine (H2NCH2CH2NH2). The pKa values for the...
Calculate the pH of a 0.368 M solution of ethylenediamine
(H2NCH2CH2NH2). The pKa values for the acidic form of
ethylenediamine ( H3NCH2CH2NH3 ) are 6.848 (pKa1) and 9.928 (pKa2).
Calculate the concentration of each form of ethylenediamine in this
solution at equilibrium.
a) Calculate the pH of a 0.045 M potassium chlorite solution. (The pKa for chlorous acid...
a) Calculate the pH of a 0.045 M potassium chlorite solution.
(The pKa for chlorous acid is 1.92)
b) Calculate the pH of a solution that is 0.35 M hydroxylamine
and 0.50 M hydroxylammonium chloride, (The pKb for
hydroxylamine is 7.96)
Calculate the pH of a solution starting with 200.0 mL of 0.010 M butanoic acid (pKa...
Calculate the pH of a solution starting with 200.0 mL of 0.010 M
butanoic acid (pKa = 4.818) solution that has been titrated to the
equivalence point with 0.050 M NaOH. Ignore activities and state
your answer with 3 sig. figs. Use approximations if you can.
Calculate the values of the hydroxide ion concentration, pOH, and pH for a 0.20 M solution...
Calculate the values of the hydroxide ion concentration,
pOH, and pH for a 0.20 M solution of ammonia
a. What is the pKa of a 0.010 M solution with a pH of 5.24? b....
a. What is the pKa of a 0.010 M solution with a pH of 5.24?
b. CaCO3 is dissolved in water. What is the complete
charge balance equation for its dissolution?
1. Calculate the pH of the buffer of a solution of 0.02M acetic acid (pKa =...
1. Calculate the pH of the buffer of a solution of 0.02M acetic
acid (pKa = 4.76) and 0.04 M acetate
2. Based on your answer to the previous question, how much of a
0.5 M solution of HCl must be added to 200 mL of the above
mentioned solution to lower the pH to 2.50? Ignore volume
changes.
calculate the pH of the following aqueous solution at 25 degreesCelsius and .35M NaF (pKa for...
calculate the pH of the following aqueous solution at 25
degreesCelsius and .35M NaF (pKa for HF=3.14)
Calculate the pH of 0.14 M RbOCl solution
Calculate the pH of 0.14 M RbOCl solution
Calculate the pH of a 0.040 M solution of CH3CO2H.
Calculate the pH of a 0.040 M solution of CH3CO2H.
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
ADVERTISEMENT
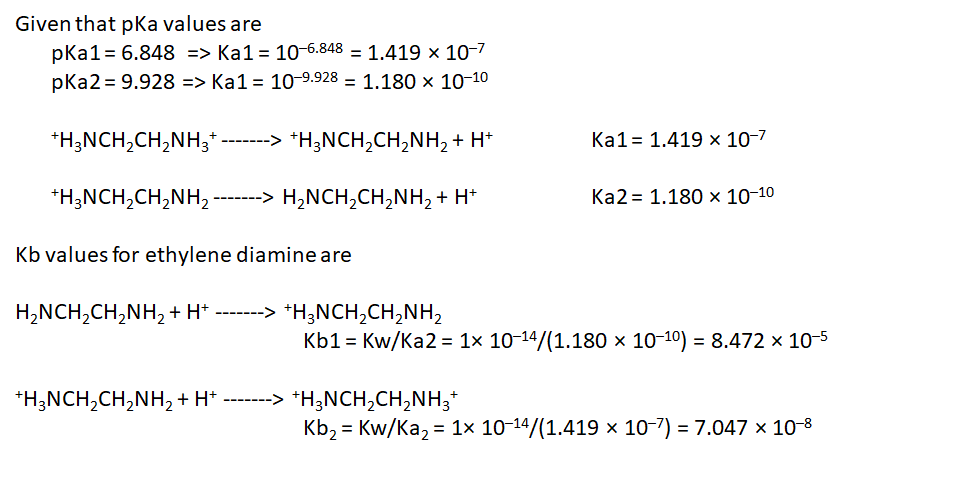

 queen_honey_blossom answered 1 month ago
queen_honey_blossom answered 1 month ago