Question
In: Chemistry
in free radical polymerization, wastage reaction are due to
in free radical polymerization, wastage reaction are due to
Solutions
Expert Solution
Ans- in free radical polymerization wastage reaction is Due to side reactions and inefficient synthesis of the radical species, chain initiation is not 100%. The efficiency factor, f, is used to describe the effective radical concentration. The maximum value of f is 1.0, but values typically range from 0.3-0.8. The following is a list of reactions that decrease the efficiency of the initiator.
- Primary recombination: Two radicals re-combine before initiating a chain. This occurs within the solvent cage, meaning that no solvent has yet come between the new radicals.
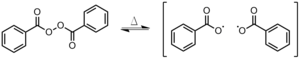
- Other recombination pathways: Two radical initiators re-combine before initiating a chain but not in the solvent cage.

- Side reactions: One radical is produced instead of the three radicals that could be produced.

Related Solutions
Compare and contrast the key characteristics of anionic polymerization and conventional free-radical polymerization
Compare and contrast the key characteristics of anionic
polymerization and conventional free-radical polymerization
What are the differences between the mechanism of free radical and coordination polymerization?
What are the differences between the
mechanism of free radical and coordination polymerization?
How "wastage reactions" can be minimized by taking into account such factors 1) radical stability, 2)...
How "wastage reactions" can be minimized by taking into account
such factors
1) radical stability, 2) radical 1/2 life, 3) monomer
reactivity?
With the aid of diagrams describe the radical polymerization of vinyl acetate using AIBN as initiator....
With the aid of diagrams describe the radical polymerization of
vinyl acetate using AIBN as initiator. Describe initiator
formation, monomer initiation, and propagation steps. In addition,
describe the various mechanisms by which chain termination (no
capacity for further growth) can occur.
Describe the various ways in which charge transfer could occur
(potential for further growth) for the polymerization described in
part (b) above.
Please explain the affects that concentration of monomer can have on typical a radical polymerization
Please explain the affects that concentration of monomer can
have on typical a radical polymerization
The living character of living radical polymerization is limited under certain conditions, such as high monomer...
The living character of living radical polymerization is limited
under certain conditions, such as high monomer conversion, high
initiator concentration, and high targeted molecular weight
(>100,000). Explain why these conditions result in broadening of
PDI and some difficulty in producing block copolymers with
well-defined block lengths of different monomers.
discuss polymerization reaction by giving examples.what are biopolymers ?
discuss polymerization reaction by giving examples.what are
biopolymers ?
Why is reaction reversibility significant in DNA polymerization?
Why is reaction reversibility significant in DNA
polymerization?
Describe why termination in radical polymerization by disproportionation and combination result in different polydispersities. Which results...
Describe why termination in radical polymerization by
disproportionation and combination result in different
polydispersities. Which results in a higher PDI?
Show mechanism for 2 methylpropane free radical monobromination. Explain why pryon undergoes substitute reaction like benzene...
Show mechanism for 2 methylpropane free radical monobromination.
Explain why pryon undergoes substitute reaction like benzene
but not addition reaction at the C=C bond
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
ADVERTISEMENT
 queen_honey_blossom answered 2 months ago
queen_honey_blossom answered 2 months ago