Question
In: Chemistry
Balancing Redox Equations in Acidic or Basic Solutions In addition to mass balance, oxidation-reduction reactions must...
Balancing Redox Equations in Acidic or Basic Solutions
In addition to mass balance, oxidation-reduction reactions must be balanced such that the number of electrons lost in the oxidation equals the number of electrons gained in the reduction. This balancing can be done by two methods. The oxidation number method balances the net increase in oxidation of the substance oxidized with the net decrease in the oxidation number of the substance reduced. The half-reaction method balances the electrons lost in the oxidation half-reaction with the electrons gained in the reduction half-reaction. In both methods
H2O(l),OH?(aq), and H+ (aq) may be added to complete the mass balance. Which substances are used depends on the reaction conditions.
Acidic solution
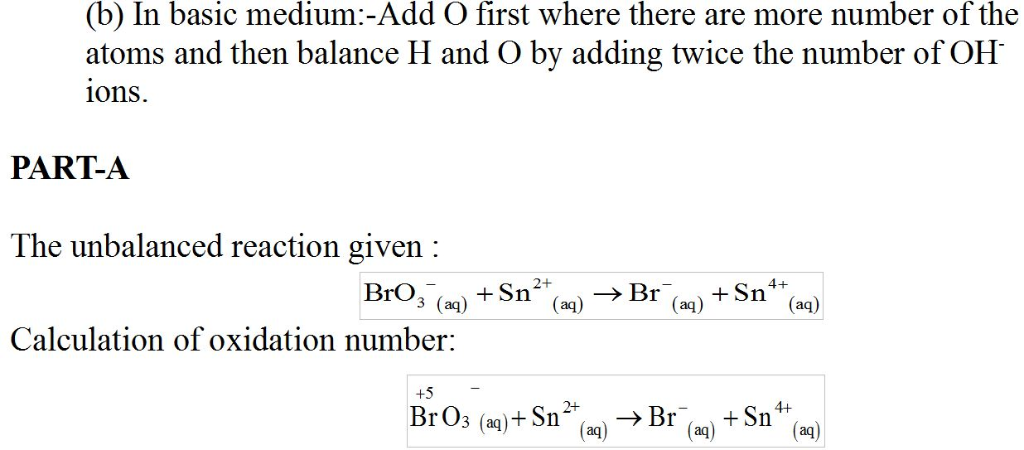
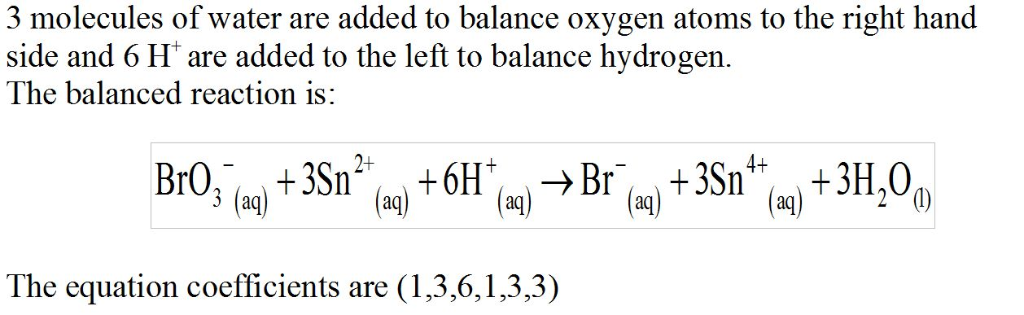
In acidic solution, bromate ion can be used to react with a number of metal ions. One such reaction is
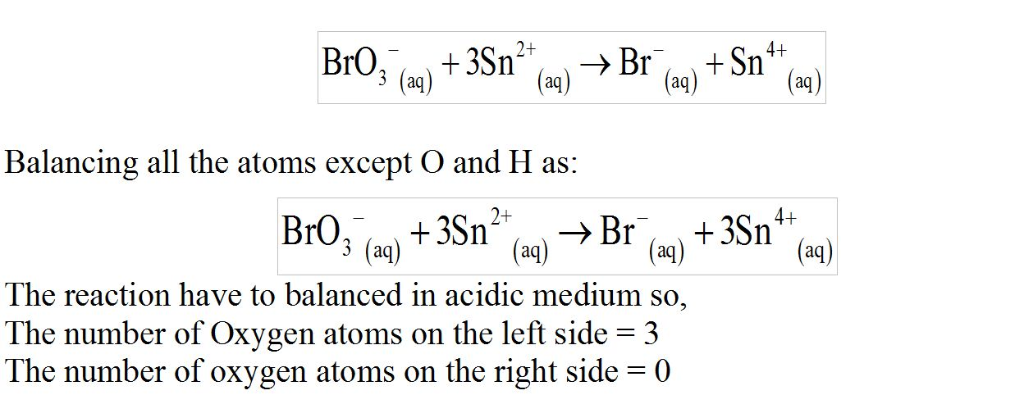
BrO3?(aq)+Sn2+(aq)?Br?(aq)+Sn4+(aq)
Since this reaction takes place in acidic solution,
H2O(l)andH+(aq) will be involved in the reaction. Places for these species are indicated by the blanks in the following restatement of the equation:
BrO3?(aq)+Sn2+(aq)+ ____ ?Br?(aq)+Sn4+(aq)+ ___
Part A
What are the coefficients of the six species in the balanced equation above? Remember to include coefficients for
H2O(l)andH+(aq)
in the appropriate blanks.
Enter the equation coefficients in order separated by commas (e.g., 2,2,1,4,4,3).
Basic solution
Potassium permanganate,
KMnO4, is a powerful oxidizing agent. The products of a given redox reaction with the permanganate ion depend on the reaction conditions used. In basic solution, the following equation represents the reaction of this ion with a solution containing sodium sulfite:
MnO4?(aq)+SO32?(aq)?MnO2(s)+SO42?(aq)Since this reaction takes place in basic solution,H2O(l)andOH?(aq)
will be shown in the reaction. Places for these species are indicated by the blanks in the following restatement of the equation:
MnO4?(aq)+SO32?(aq)+ ____?MnO2(s)+SO42?(aq)+ ___
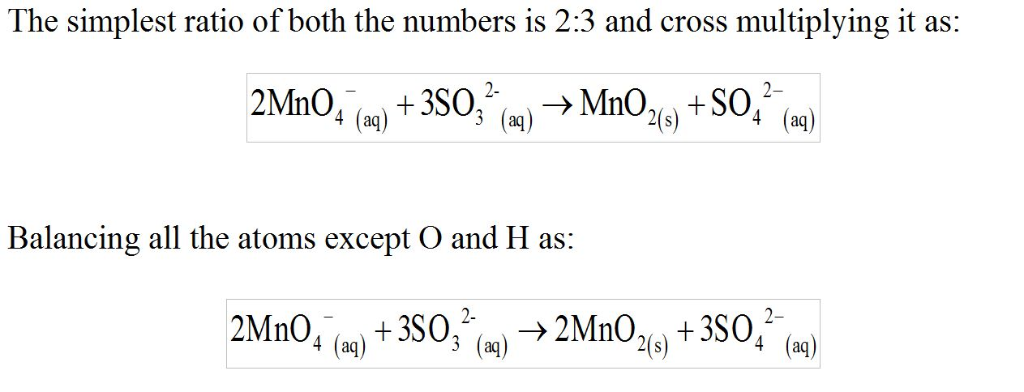
Part B
What are the coefficients of the six species in the balanced equation above? Remember to include coefficients for
H2O(l)andOH? (aq) in the blanks where appropriate.
Enter the equation coefficients in order separated by commas (e.g., 2,2,1,4,4,3).
Solutions
Related Solutions
In addition to mass balance, oxidation-reduction reactions must be balanced such that the number of electrons...
Redox (oxidation-reduction) reactions in glycolysis
Why must the charge balance in oxidation-reduction reactions?
Using half equations for the oxidation and reduction, develop the redox equation for the oxidation of...
Balance the following redox reactions. Show the complete balanced reduction half and oxidation half reaction, and...
Separate the following redox reactions into half-reactions, and label each half-reaction as oxidation or reduction. Part...
Continue and balance the following reactions. Provide half-reactions for balancing of redox processes P4 + KOH...
2. Balance the first redox reaction in acidic solution and the second redox reaction in basic...
Balance the following oxidation–reduction equation. The reactions occur in a basic aqueous solution. Cd2++ H 2S → Cd...
use oxidation numbers to decide which of the following are redox reactions. for all redox reactions...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...


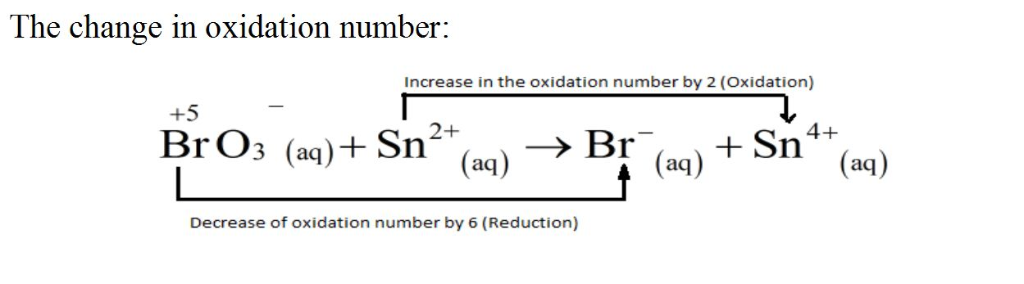
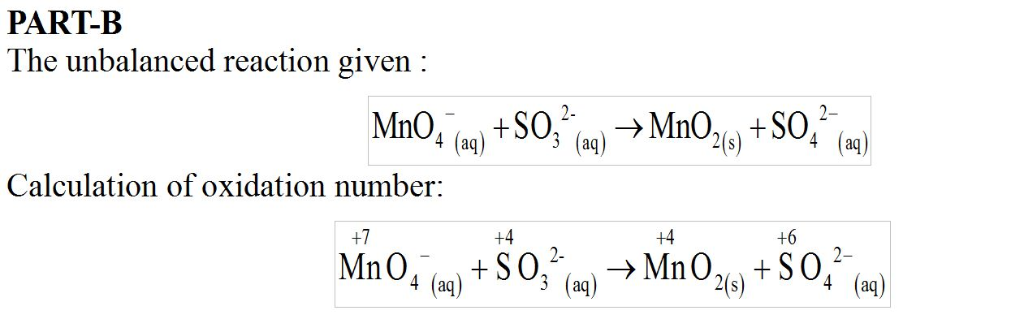
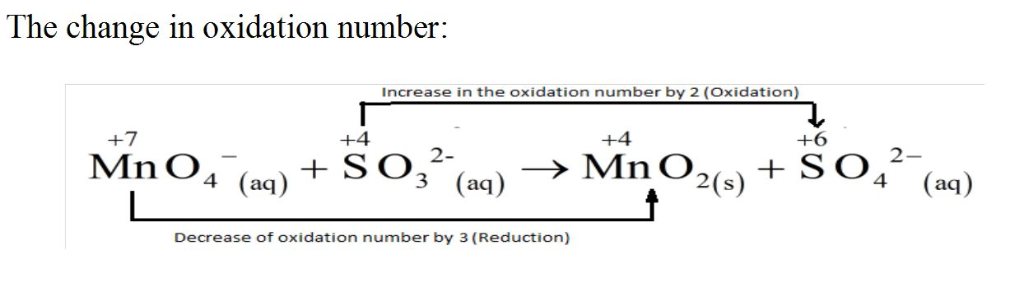
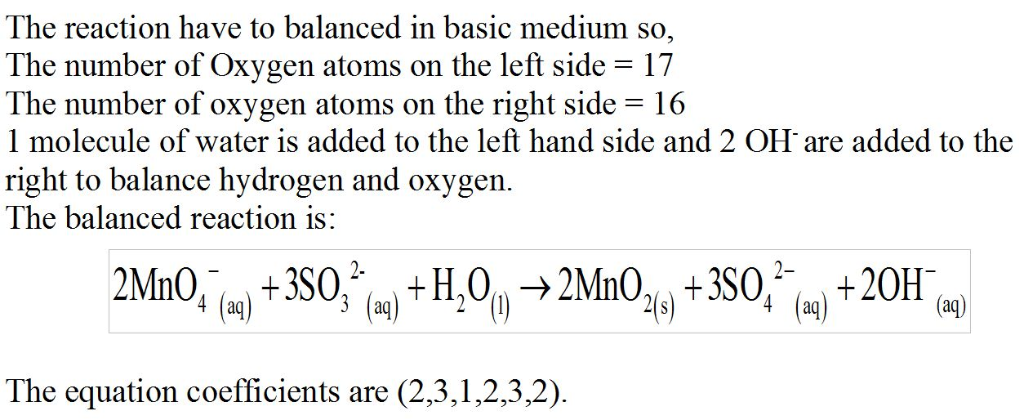
 queen_honey_blossom answered 2 months ago
queen_honey_blossom answered 2 months ago