Question
In: Chemistry
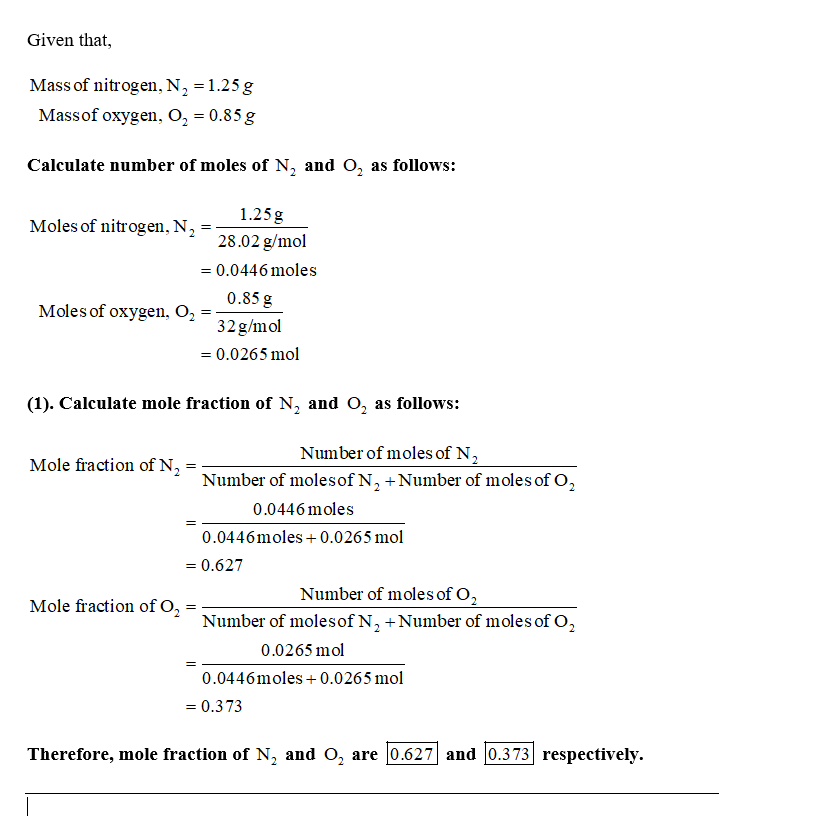
A gas mixture contains 1.25g N2 and .85g O2 in a 55 L container at 18...
A
gas mixture contains 1.25g N2 and .85g O2 in a 55 L container at 18
degrees Celsius. Calculate the mole fraction and the partial
pressure of each component in the gas mixture.
Solutions
Related Solutions
A gas mixture contains 1.25 g N2 and 0.84 g O2 in a 1.53-L container at...
A gas mixture contains 1.25 g N2 and 0.84 g O2 in a 1.53-L
container at 14 ∘C.
A.) Calculate the mole fraction of N2.
B.) Calculate the mole fraction of O2.
C.) Calculate the partial pressure of N2.
D.) Calculate the partial pressure of O2.
A gas mixture contains 5 kg of N2 and 10 kg of O2. Determine (a) the...
A gas mixture contains 5 kg of N2 and 10 kg of
O2. Determine (a) the average molar mass and (b) gas
constant. (c) What-if Scenario:What would the
average molar mass be if the gas mixture contained 10 kg of
N2 and 5 kg of O2?
a) ? kg/mol
b)? KJ/kgk
c) ? kg/mol
An ideal gas mixture contains 220 mg N2 and 110 mg of O2. If the partial...
An ideal gas mixture contains 220 mg N2 and 110 mg of O2. If the
partial pressure of O2 at 27 oC is 19.8 kPa determine
(a) The total pressure
(b) The molar volume of the mixture
(c) The collision frequency, (i) zN2-O2 (ii) zO2-N2
(d) The mean free path of both gases
(e) The collision density (i) ZN2-O2 (ii) ZO2-N2
Given, the collision diameters of N2 and O2 are 3.75 and 3.61 Å,
respectively.
If a gas mixture at 298 K, which contains 90% N2, 5% H2O, and 5% O2,...
If a gas mixture at 298 K, which contains 90% N2, 5% H2O, and 5%
O2, is instantaneously heated to 2000 K, plot the NO concentration
as a function of time.
Plot the NO concentration as a function of time, if the mixture
is heated to 3000K.
A gaseous mixture of O2 and N2 contains 40.8 % nitrogen by mass. What is the...
A gaseous mixture of O2 and N2 contains 40.8 % nitrogen by mass.
What is the partial pressure of oxygen in the mixture if the total
pressure is 385 mmHg ? Express you answer numerically in
millimeters of mercury.
A reaction container contains 10.7 atm of both N2 and O2 and 5.97 atm of NO....
A reaction container contains 10.7 atm of both N2 and
O2 and 5.97 atm of NO. What is the pressure of NO once
equilibrium is established? Kp = 2.4 x
10-2
N2 (g) + O2(g) ⇌ 2 NO (g)
Show your work!
A gas mixture contains 20.0 mole% H2O(v) and 80.0 mole% N2. The gas temperature and absolute...
A gas mixture contains 20.0 mole% H2O(v) and 80.0
mole% N2.
The gas temperature and absolute pressure at the start of each of
the three parts of this problem are 66°C and 700.0 mm Hg.
Ideal gas behavior may be assumed in every part of this
problem.
a. If some of the gas mixture is put in a cylinder and slowly
cooled at constant pressure, at what temperature would the first
drop of liquid form? °C
b. If a 30.0...
A gas mixture contains 20.0 mole% H2O(v) and 80.0 mole% N2. The gas temperature and absolute...
A gas mixture contains 20.0 mole% H2O(v) and 80.0 mole% N2. The
gas temperature and absolute pressure at the start of each of the
three parts of this problem are 71°C and 700.0 mm Hg. Ideal gas
behavior may be assumed in every part of this problem.
a. If some of the gas mixture is put in a cylinder and slowly
cooled at constant pressure, at what temperature would the first
drop of liquid form? °C
b. If a 30.0...
A gas mixture contains the following gases at the indicated partial pressures: N2, 310. torr ;...
A gas mixture contains the following gases at the indicated
partial pressures: N2, 310. torr ; O2, 144 torr ; and He, 225 torr.
1. What mass of each gas is present in a 2.85 L sample of this
mixture at 25.0 ∘C?
A gas mixture contains the following gases at the indicated partial pressures: N2, 310. torr ;...
A gas mixture contains the following gases at the indicated
partial pressures: N2, 310. torr ; O2, 144 torr ; and He, 225 torr.
1. What mass of each gas is present in a 2.85 L sample of this
mixture at 25.0 ∘C?
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- C PROGRAMMIMG I want to check if my 2 input is a number or not all...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
ADVERTISEMENT

 queen_honey_blossom answered 2 months ago
queen_honey_blossom answered 2 months ago