Question
In: Chemistry
how to put formulate the question : Calculate the number of atoms present in 3.752g sample...
how to put formulate the question :
Calculate the number of atoms present in 3.752g sample of
Pb?
Solutions
Expert Solution
Answer :- 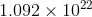 atom of Pb
atom of Pb
----------------------------
Explanation :-
Given :- Mass of Pb = 3.752 g
we know, Molar mass of Pb = 207 g/mol
To calculate number of atoms present in 3.752 g Pb, we first calculate number of moles of Pb in 3.752 g and then this number of moles is multiply by Avogadro's number.
Formula :-
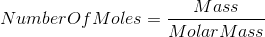
Therefore,
mole of Pb = 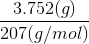 = 0.01813 mol
= 0.01813 mol
we know that,
According to Avogadro's law,
1 mol of substance = 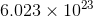 atoms of substance
atoms of substance
Thus, we have,
1 mol of Pb = 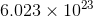 atoms of Pb
atoms of Pb
therefore,
0.01813 mol of Pb =
= 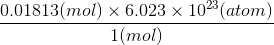
= 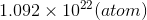 of Pb
of Pb
---------------------------------------------------------------
Or
we can also formulate it as
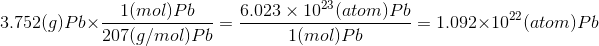
Related Solutions
.Calculate the number of copper atoms in a 63.55 g sample of copper. .What is the...
1. Using average atomic masses calculate the number of atoms present in each of the following...
For the given elements, calculate the number of atoms in 1.00 gram of that element. How...
Use Avagadros number to calculate the number of molecules of H2S, the number of atoms of...
Calculate the number of oxygen molecules and the number of oxygen atoms in 30.5 g of...
To calculate the number of atoms in a face centered cubic cell?
Calculate the diameter of a lead sphere that contains the same number of atoms as a...
Part A Calculate the number of C atoms in 0.602 mole of C. Part B Calculate...
Calculate the number of atoms per cubic meter in Cobalt (Co) (units atoms/m3). Write your answer...
Calculate each of the following. Part A number of Ag atoms in 0.125 mole of Ag...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
 queen_honey_blossom answered 2 months ago
queen_honey_blossom answered 2 months ago