Question
In: Chemistry
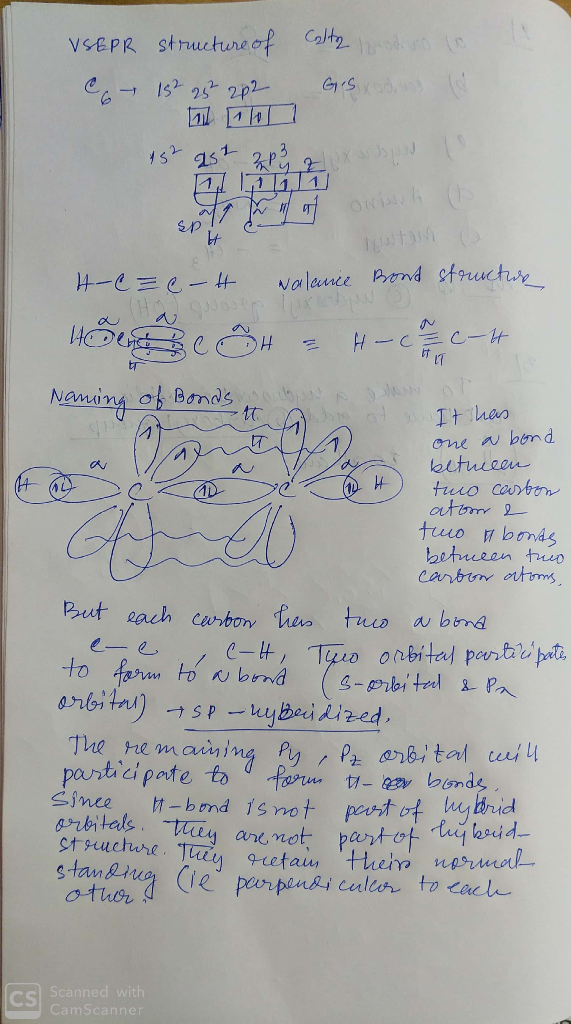
draw vsepr and valence bond picture for C2H2? label the bonds. for each sigma bond name...
draw vsepr and valence bond picture for C2H2? label the bonds. for each sigma bond name the two orbitals that overlap to form it? for each pi bond name the two orbitals that overlap to form it. why are to pi ponds perpindicular to each other. why are quadruple bonds unlikely? Please label thank you.
Solutions
Related Solutions
Draw the VSEPR picture for, C2H2, HNNH (one hydrogen on each nitrogen), ClO3-, NO3-, PCl3 and...
Draw the VSEPR picture for, C2H2, HNNH
(one hydrogen on each nitrogen), ClO3-, NO3-, PCl3 and
Identify the electron pair geometry, the molecular geometry and the
bond angles.
Draw a simple picture and label amylose, amylopectin, and glycogen. Describe the unique properties of each...
Draw a simple picture and label amylose, amylopectin,
and glycogen. Describe the unique properties of each
polysaccharide, and where these are found.
Draw a picture of the bacterial growth curve chart. Label each section and describe what is...
Draw a picture of the bacterial growth curve chart. Label each
section and describe what is occurring in each section.
Draw the Lewis structure, VSEPR formula, molecular shape, and bond angles for each of the following...
Draw the Lewis structure, VSEPR formula, molecular shape, and
bond angles for each of the following species:
A) SiCl4
B) PF5
C) SBr2
D) ICl2+
Draw a picture of a cell located in the intestines for yourself and label the surfaces...
Draw a picture of a cell located in the intestines for yourself
and label the surfaces and draw the location of the different
receptors found on the plasma membranes. Then explain in your own
words to turn in all of the different carrier proteins involved in
the reabsorbtion of sodium and glucose from the intestinal mucosa
to the blood.
What is the functional significance of tight junctions? Reflect
on the fact that this is the same system involved in the...
Draw the VSEPR structure for XeO2F2. Give the name of the molecular shape and indicate the...
Draw the VSEPR structure for XeO2F2. Give the name of the
molecular shape and indicate the bond angles.
Draw the VSEPR picture for (1) CO2, (2) CH2O, (3) CH4, (4). C2H6, and Identify the,...
Draw the VSEPR picture for (1) CO2, (2) CH2O, (3) CH4, (4).
C2H6, and Identify the,
(A) electron pair geometry, (B) the molecular geometry and (C).
the bond angles.
Draw a picture of a separatory funnel with an aqueous layer and a dichloromethane layer. label...
Draw a picture of a separatory funnel with an aqueous layer and
a dichloromethane layer. label each layer. which of the two layers
would contain eugenol?
Why is the drying agent added to the dichloromethane layer after
extraction?
why is steam distillation used to isolate the eugenol from the
cloves?
Draw the Lewis structure of the conjugate acid of CH2NH. Then, draw the valence bond (bonding...
Draw the Lewis structure of the conjugate acid of CH2NH. Then,
draw the valence bond (bonding orbital) diagram for the conjugate
acid of CH2NH. How many sigma bonds are in this molecule? How many
π bonds are in this molecule? How would you describe the bonding
orbital overlap between the carbon atom and the nitrogen
atom?
Ethane (C2H6), ethylene (C2H4), acetylene (C2H2). Give the lewis structure, VSEPR shape, bond angles, molecular dipole,...
Ethane (C2H6), ethylene (C2H4), acetylene (C2H2). Give the lewis
structure, VSEPR shape, bond angles, molecular dipole, valence bond
sketch, hybridization of each carbon atom, sigma and pi bonds for
each of the three substances above.
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
ADVERTISEMENT

 queen_honey_blossom answered 3 months ago
queen_honey_blossom answered 3 months ago