Question
In: Other
1- Explain the qualitative differences between the Gibbs free energy and the chemical potential. 2- Explain...
1- Explain the qualitative differences between the Gibbs free energy and the chemical potential.
2- Explain what is wrong with the following statement: If two (or three) phases of a pure material have the same chemical potential, temperature, and pressure, then the equilibrium state of the system will allow a phase transition between the two (or three) phases.
3- Explain qualitatively the differences between Raoult's law and Henry's law, including the composition range in which they are valid.
Solutions
Expert Solution
1- Difference between Gibbs free energy and Chemical potential:
- Gibbs free energy can be intensive property associated with the pure substances while chemical potential is an extensive gibbs free energy associated with gas mixture, solutions and liquid mixtures and solutions.
- Gibbs free energy is pure substance property while chemical potential is a partial molar property
- Gibbs free energy is a function of temperature and pressure while chemical potential is function of temperature, pressure and number of moles of each component present in the mixture or solution.
2) If two phases have the same chemical potential, temperature and pressure, they are said to be in equilibrium. Since all the variables are fixed, the system cannot for allow phase transition. For example, in the below phase diagram, at fixed temperature and pressure and chemical potential, the composition of the water is uniquely specified. Hence, it cannot undergo phase transition, However, if the pressure is not fixed, it transition from solid to liquid or liquid to vapor and so on by changing pressure.
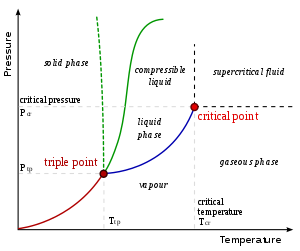
3) 

Related Solutions
I have a question about gibbs free energy vs gibbs standard free energy. We know that...
Consider the following reaction and calculate the standard Gibbs Free Energy for it. 2 NO +...
Explain your answer. Internal Energy (U); Enthalpy (H); Helmholtz Free Energy (A or F); Gibbs Free...
Predict the standard cell potential and calculate the standard reaction Gibbs free energy for galvanic cells...
verify the maxwell relations associated with the helmholtz free energy F and the Gibbs free energy...
Derive the Maxwell equations for F (Helmholtz Free Energy) and G (Gibbs Free Energy) with “for...
Explain the chemical reason for the difference in calories (measure of chemical potential energy) in a...
what is the difference between the Gibbs energy of a reaction mixture and the Standard Gibbs...
In a certain chemical reaction, there is a decrease in the potential energy (chemical energy) as...
Chemical potential energy vs. kinetic energy Know the difference between these two energy types. Energy...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
- For this assignment you will write a program with multiple functions that will generate and save...
- How many grays is this?Part A A dose of 4.7 Sv of γ rays in a...
- how to operate a business?
- Discuss pros and cons of using twins to estimate the rate of return to school
 Amanda K answered 3 years ago
Amanda K answered 3 years ago