Question
In: Physics
What is the energy needed to cause an electron to undergo a transition from the n...
What is the energy needed to cause an electron to undergo a transition from the n = 4 state to the n = 5 state in hydrogen?
Solutions
Expert Solution
energy needed to cause an electron to undergo a transition from the n = 4 state to the n = 5 state in hydrogen =
=> 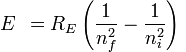
=> -2.178 x 10-18( 1/25 - 1/16)
=>0.04* 10-18 j
Related Solutions
14: An electron in hydrogen atom at the energy level n = 7 undergo a transition...
14: An electron in hydrogen atom at the energy level n = 7
undergo a transition to level n = 3: Find the frequency and the
energy of the emitted photon.
15: An electron jumps from higher energy level to the first
energy level with an energy difference of 2.04375 x
10-18 J. find the initial energy level. Show your
calculations,
16: A: What will be the speed of an electron at 4 th energy
level? Suppose the electron has...
Consider the transition from energy levels n = 1 to n = 3 a) what is...
Consider the transition from energy levels n = 1 to n = 3
a) what is the frequency and
wavelength associated with this transition?
b) in what spectral region does this
transition occur?
c) is energy absorbed?
9) What type of electron orbital (i.e.
s, p, d, or f) is designated by
a) n = 2, l = 1,
ml = -1?
b) n = 1, l = 0,
ml = 0?
c) n = 5, l =...
The transition of an electron from the principle quantum number n = 4 to n =...
The transition of an electron from the principle quantum number
n = 4 to n = 2 in atomic hydrogen causes emission of a photon of
what wavelength?
Ionization energy is the energy needed to eject an electron from an atom or ion. Calculate...
Ionization energy is the energy needed to eject an electron from
an atom or ion. Calculate the ionization energy, IE, of the
one-electron ion Be3 . The electron starts in the lowest energy
level, n=1.
Answer the following: a. Find the energy needed to excite an electron in hydrogen from the...
Answer the following:
a. Find the energy needed to excite an electron in hydrogen from
the ground state to the n=5 excited state.
b. What is the wavelength and frequency of the photon emitted
when this electron relaxes to the n=2 state?
c. How much energy would it take to remove an electron from
hydrogen? (transition from n=1 to n=∞)
After an electron undergoes a n to π2 or π to π2 transition, what are three...
After an electron undergoes a n to π2 or π
to π2 transition, what are three ways we discussed it
will “relax” or “lose this excess energy”?
And what determines if a transition such as those described in
question 1 will take place? (another way to ask this might be, why
don’t other bond types also undergo a transition – or, do
they?).
1.) Determine the energy change (in Joules) associated with the transition from n=2 to n=4 in...
1.) Determine the energy change (in
Joules) associated with the transition from n=2 to n=4 in the
Hydrogen atom.
2.) Calculate the wavelength (in nm)
of the red line in the visible spectrum of excited
H atoms using Bohr Theory.
3.) What is the energy (in Joules) of
the X-ray radiation at 2.00x10^-10 m that are used for medical
diagnosis?
4.) What is the binding energy of an
electron in a photosensitive metal (in kj/mol) if the minimum
frequency of...
Calculate the energy change when an electron falls from the n =6 energy level to the...
Calculate the energy change when an electron falls from the n =6
energy level to the n = 1 energy level in a hydrogen atom.
ΔE=−2.178×10−18 J ( 1/n^2 final)- (1/n^2 initial)
How many orbitals are described by each of the below
combinations of quantum numbers?
n = 3, ℓ =2
n = 4, ℓ = 2, mℓ = 2
Choose one:
A. orbitals that randomly fill, but that are always
identical.
B. orbitals of the same shape.
C. orbitals...
Calculate the photon energy and wavelength for the n = 4 to n = 2 transition...
Calculate the photon energy and wavelength for the n = 4 to n =
2 transition and n = 4 to n = 3 transition for the hydrogen atom, H
(Z = 1). Do not include uncertainty.
E ( 5--> 3) =
E ( 5 --> 2 ) =
λ ( 5--> 3 ) =
λ ( 5 --> 2) =
What minimum amount of electromagnetic energy is needed to produce an electron and a positron together?...
What minimum amount of electromagnetic energy is needed to
produce an electron and a positron together? A positron is a
particle with the same mass as an electron, but has the opposite
charge.
E=?
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
ADVERTISEMENT
 genius_generous answered 7 months ago
genius_generous answered 7 months ago