Question
In: Biology
You are presented with 10 millileters of a proteins solution that is at a concentration of...
You are presented with 10 millileters of a proteins solution that is at a concentration of 100 millimolars. You dilute the solution by taking 1 microliter of the solution putting it in an empty test tube then add adding 99 ul of buffer for your experiment. What is your final concentration of the peptide solution that is in this test tube.
*The answer key says that the answer is 10^-3 M*
If this is the answer can you please show work. Thank you.
Solutions
Expert Solution
Answer-
1. We have 10ml of protein solution with concentration 100mM. If
we dilute the solution by taking 1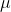 l
of solution and putting it in an empty test tube and then
99
l
of solution and putting it in an empty test tube and then
99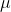 l
of buffer is added then the final concentration of of the peptide
solution in test tube is-
l
of buffer is added then the final concentration of of the peptide
solution in test tube is-
Dilution can be calculated by formula-
C1V1=C2V2
Where, C1= initial concentration of protein solution (100mM)
V1= volume taken from stock solution (1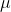 l)
l)
C2= final concentration of protein solution
V2= volume of working solution in test tube (100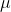 l)
l)
By putting these values-
C1V1=C2V2
100mM×1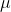 l=C2×100
l=C2×100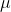 l
l
C2= 1×100/100
C2=1mM = 10-3M( as 1M=1000mM)
The final concentration of the peptide solution in test tube is 1mM i. e. 10-3M.
Related Solutions
I) You have an HCl solution of pH 3.13. (a) The concentration of this solution is...
A back-titration was peformed as follows: In a solution with an unknown concentration of nickel, 10...
An unknown solution containing iron at a concentration between 10 and 50 mg/L is now to...
Calculate the concentration and the pH in a .10 M solution of sodium acetate, Na(CH3CO2). Equilibrium...
You want to determine the concentration of Fe2+in a solution. You decide to determine the Fe2+...
Calculate the concentration of the standardized thiosulfate solution. The following are the steps and the concentration...
You need to determine the concentration of a sulfuric acid solution by titration with a standard...
You need to determine the concentration of a sulfuric acid solution by titration with a standard...
how do you calculate the stock concentration of a chemical solution in a bottle.
Part A Calculate the molar concentration of OH− ions in a 8.1×10−2 M solution of ethylamine...
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
 gladiator answered 1 year ago
gladiator answered 1 year ago