Question
In: Chemistry
This is for a titration experiment starting with 25 ml of HCL. I've answered two but...
This is for a titration experiment starting with 25 ml of HCL. I've answered two but am struggling with the others.
2. Find the equivalence point on the graph. What is the equivalence volume of NaOH at this point?
The pH at the equivalence point is 8.77 and the volume of NaOH at this point is 40.00 mL of NaOH
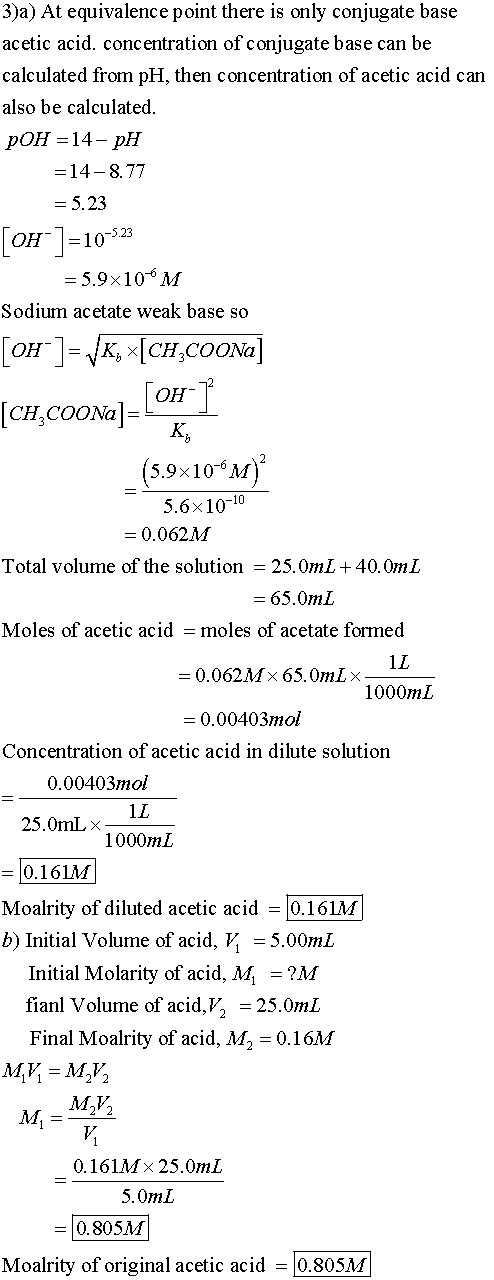
3. a. Calculate the unknown molarity of the diluted acetic acid from the volumes of acid and base at the equivalence point and the molarity of the NaOH Ma × Va = Mb × Vb.
b. Once you find the molarity of your diluted solution use that to
calculate the molarity of the original solution using the equation
M1 × V1 = M2 × V2 a
second time.
4. In experiment 1, you were able to calculate the concentration of the HCl solution using the initial pH. Would this same approach work with the acetic acid? Why or why not?
Solutions
Expert Solution
4. Same approch does not work for acetic acid. Because acetic acid is weak acid and it is not dissociated completely as ions. We can calculate the concentration of acetic acid from initial pH and Ka of acetic acid. But it is different approach.
Related Solutions
In the titration of 25 mL of 0.100 M N2H2 (Kb=8.9x10-7) with .100M HCl, determine the...
In this experiment, you will mix 1 mL of 1 M HCl with 9 mL of...
A student conducts a titration of 50 mL of HCl with 1.00 M NaOH. The pH...
For the titration of 25.00 mL of 0.1000 M HCl with 0.1000 M NaOH, calculate the...
In a titration of 200 ml of 0.150 M HCl with 0.200 M KOH, what is...
Calculate the pH in the titration of 25.0 mL of 0.100M HCl with 0.200M NaOH. Finally,...
Calculate the pH in the titration of 25.0 mL of 0.100M HCl with 0.200M NaOH. Next,...
Consider the titration of 25.0 mL of aqueous sample of methamphetamine (C10H14NH) with 0.0955M HCl. It...
Calculate the pH in the titration of 20 mL of 0.125 M HCL with 0.250 M...
consider the titration of a strong acid, HCL, with a strong base NaOH. The 35 ml...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 queen_honey_blossom answered 1 year ago
queen_honey_blossom answered 1 year ago