Question
In: Other
1. Consider a simplified one-dimensional laminar flame, such as that discussed in the classroom. The earliest...
1. Consider a simplified one-dimensional laminar flame, such as that discussed in the classroom. The earliest description of a laminar flame is that of Mallard and Le Chatelier in 1883. Assume that:
a. 1-D, constant area, steady flow,
b. kinetic and potential energies, viscous shear work, and thermal
radiation are neglected,
c. pressure is constant,
d. diffusion of heat and mass are governed by Fourier’s and Fick’s laws
and binary diffusion is applied,
e. Lewis number is unity,
f. individual specific heats are all equal and constant,
g. fuel and oxidizer form products in a single-step exothermic reaction,
h. fuel is completely consumed at the flame with oxidizer in
stoichiometric or excess proportion.
By applying suitable boundary conditions, please derive the expressions for laminar flame velocity and flame thickness. (Simplified solution can be found in most of the combustion textbook, such as the one by S.R. Turns(1996) or the one by I. Glassman (1993 or newer edition).)
Solutions
Expert Solution
1) Mass Conservation equation

Hence, 
 is
the mass flux
is
the mass flux
Species conservation equation

Change in mass flux is balanced by change in mass due to chemical reaction

 is the mass fraction of species,
is the mass fraction of species,  is mass diffusivity,
is mass diffusivity,
 is
mass produced due to chemical reaction
is
mass produced due to chemical reaction
We balance the stoichiometric equation
1 units of fuel is mixed with  units of oxidizer
to give
units of oxidizer
to give  of products
of products
Hence,

Energy conservation equation
 ----------------------(1)
----------------------(1)
where  is the heat produced due the exothermal equation and is the sum of
the heat capacity of individual species
is the heat produced due the exothermal equation and is the sum of
the heat capacity of individual species
Hence,

Since, we can take out  as common and club all other individual heat capacities
as common and club all other individual heat capacities 
Now, Lewis number is 1 which means mass diffusivity to heat diffusivity is same or

Putting above relation in eq (1), we get
 ----------------------(2)
----------------------(2)
The flame velocity can be calculated from its mass flux by
 where S_c is flame velocity
where S_c is flame velocity
Boundary conditions
Suppose the flame has a thickness 
Upstream Downstream




Integrating equation (2), we get
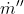

now converting the spatial variable in integration to temperature variable


Now we define the average reaction rate or temperature averaged mass production as

Hence, the energy conservation equation becomes
 --------------------(3)
--------------------(3)
Above equation has two unknowns, so we have to make an assumption here. We assume that the rate of reaction is slow in upstream than in downstream, then original energy equation becomes




Putting \delta in eq (3)

We know flame speed  and
and
flame thickness 
From  , we
can calculate both flame speed and flame thickness
, we
can calculate both flame speed and flame thickness
Related Solutions
1. A premixed stoichiometric methane-air flame has a laminar flame speed of 0.33 m/s and a...
1. A premixed stoichiometric methane-air flame has a laminar flame speed of 0.33 m/s and a...
Consider the two-dimensional laminar boundary layer flow of air over a wide 15 cm long flat...
Consider the Hamiltonian of a particle in one-dimensional problem defined by: H = 1 2m P...
Consider a particle that is confined by a one dimensional quadratic (harmonic) potential of the form...
Consider a one-dimensional lattice with a basis of two non-equivalent atoms of masses M_1and M_2. 1....
Consider a particle of mass m confined to a one-dimensional box of length L and in...
Consider the one-dimensional motion of a particle of mass ? in a space where uniform gravitational...
Consider a particle of mass m that can move in a one-dimensional box of size L...
Consider the one dimensional model of one-particle-in-a-box. Under what condition the two quantum levels are orthogonal....
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 Amanda K answered 3 years ago
Amanda K answered 3 years ago