Question
In: Chemistry
Explain why each change in conditions would affect the reaction rate of reaction when sodium metal...
Explain why each change in conditions would affect the reaction rate of reaction when sodium metal reacts with chlorine gas to form NaCl?
Choose from the options:
A. more collisions occur between the atoms
B. fewer collisions occur between the atoms
C. more collisions and more energy occur between the atoms
D. fewer collisions and less energy occur between the atoms
E. reduces the activation energy needed
F. increases the activation energy needed
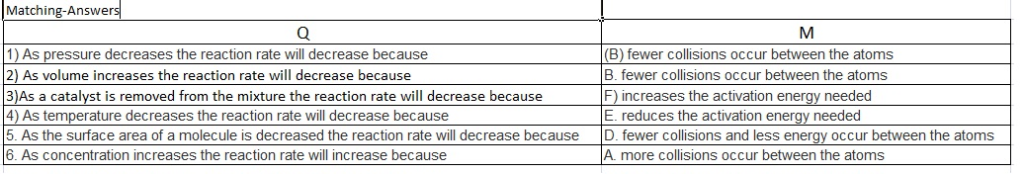
1. As pressure decreases the reaction rate will decrease because
2. As volume increases the reaction rate will decrease because
3. As a catalyst is removed from the mixture the reaction rate will decrease because
4. As temperature decreases the reaction rate will decrease because
5. As the surface area of a molecule is decreased the reaction rate will decrease because
6. As concentration increases the reaction rate will increase because
Solutions
Expert Solution
According to Collision's theory, the left side statements that suit to the right side are as follows.
Related Solutions
What is the change in enthalpy (in kJ) under standard conditions when 233.71 g of sodium...
What is the change in enthalpy (in kJ) under standard conditions when 200.18 g of sodium...
Explain why unfavorable economic or political conditions affect the MNC’s cash flows, required rate of return,...
1. State and Explain how each of these errors would affect (high, low, no change) your...
Match how each of the following will change the rate of this forward reaction.
With the goal of stabilizing output, explain how and why you would change the interest rate...
what happens when any of the determinants of components of GDP change? explain why each step...
Explain how each of the Determinants of Price Elasticity of Demand would or would not affect...
ii) If the exchange rate of dollar stays fixed, how would this change affect us domestic...
For the reaction shown below, which change in conditions made to the system at equilibrium will...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago