Question
In: Chemistry
In a sealed container, 230.0 L of air was collected at 50.0°C and 652 mmHg. The...
In a sealed container, 230.0 L of air was collected at 50.0°C and 652 mmHg.
The mole fraction of oxygen gas in air is 0.052.
Calculate the density of the oxygen gas in air under these conditions.
Calculate the mass of oxygen gas in the sample.
Calculate the volume of oxygen gas of the sample.
Solutions
Expert Solution
Hii,
Please find the detailed explaination below.
EXPLAINATION :


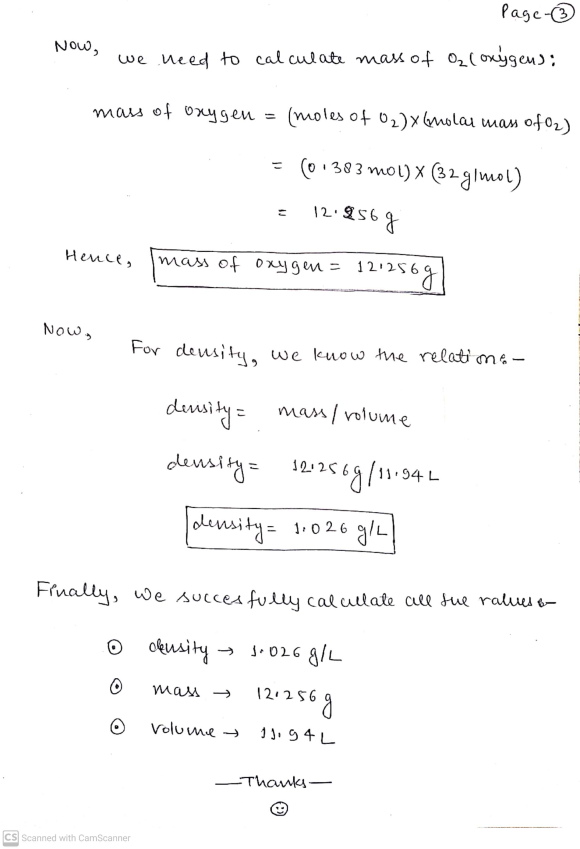
Hope, This helps you alot for your better understanding.
Thanks
?—BEST OF LUCK—?
Related Solutions
A sealed container holding 0.0255 L of an ideal gas at 0.993 atm and 67 °C...
A sealed container holding 0.0255 L of an ideal gas at 0.993 atm
and 67 °C is placed into a refrigerator and cooled to 45 °C with no
change in volume. Calculate the final pressure of the gas.
What is the mass of propane, C3H8, in a 50.0 L container of the gas at...
What is the mass of propane, C3H8, in a 50.0 L container of the
gas at STP?
An ideal gas in a sealed container has an initial volume of 2.55 L. At constant...
An ideal gas in a sealed container has an initial volume of 2.55
L. At constant pressure, it is cooled to 20.00 °C where its final
volume is 1.75 L. What was the initial temperature?
A sample of xenon gas occupies a volume of 9.47 L at 403 K. If
the pressure remains constant, at what temperature will this same
xenon gas sample have a volume of 6.29 L?
4.60 mol of solid A was placed in a sealed 1.00-L container and allowed to decompose...
4.60 mol of solid A was placed in a sealed 1.00-L container and
allowed to decompose into gaseous B and C. The concentration of B
steadily increased until it reached 1.10 M, where it remained
constant.
A(s) <===> B(g) + C(g)
Then, the container volume was doubled and equilibrium was
re-established. How many moles of A remain?
4.60 mol of solid A was placed in a sealed 1.00-L container and allowed to decompose...
4.60 mol of solid A was placed in a sealed 1.00-L container and
allowed to decompose into gaseous B and C. The concentration of B
steadily increased until it reached 1.20 M, where it remained
constant.
A (s) <---> B (g) + C (g)
Then, the container volume was doubled and equilibrium was
re-established. How many moles of A remain?
Please break down the steps so I can see how to solve. Thank
you!
1) An organism is put in a sealed container. The oxygen levels in the container increase...
1) An organism is put in a sealed container. The oxygen levels
in the container increase and the carbon dioxide levels decrease.
What can you conclude about what type of organism it is?
2) Use these eight terms to fill in the blanks:
carbon dioxide, carbon dioxide fixation, G3P, glucose, PGA,
Rubisco, RUBP, the Calvin Cycle.
When plants make sugar, they use a group of enzymes found in the
stroma to carry out a series of chemical reactions called
____________________________________....
50 L of a gas were collected over water when the barometer read 684.0 mmHg and...
50 L of a gas were collected over water when the barometer read
684.0 mmHg and the temperature was 14 oC . What volume would the
dry gas occupy at standard conditions?
HINT: Consider Dalton’s law of partial pressures.
A rigid, sealed container of R134a initially at -30°C and 40% quality is placed inside a...
A rigid, sealed container of R134a initially at -30°C and 40%
quality is placed inside a room so that it heats up at 5°C/hr. If
there is 3 kg of R134a in the container, determine:
a) The volume of the container
answer must be 0.2723 m3
b) The specific volume of the vapour when the R134a finishes
converting into a gas
answer must be 0.09075 m3/kg
c) The pressure when all the R134a has finished converting into
a gas
answer...
Consider a sealed container at 1 atm total pressure and 20 degrees C and containing both...
Consider a sealed container at 1 atm total pressure and 20
degrees C and containing both liquid and gas including air and a
solution of 10 ppm chloroform in water. Use a water vapor pressure
of 17.5 mmHg and a dimensionless concentration Henry's constant for
chloroform of 0.120.
(a) What will be the concentration of chloroform in the gas
phase? Express the answer in both ppmv and mg/m3.
Ans. _______________ ppmv
_______________ mg/m3
(b) A vacuum pump is connected to...
4 liter sealed container contains water vapour at 0.4 atm and 90°C. a) What is the...
4 liter sealed container contains water vapour at 0.4 atm and
90°C. a) What is the dew point of the vapour? b) Determine the
percentage of the vapour that will condense if the system is cooled
to 50°C.
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
ADVERTISEMENT
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago