Question
In: Chemistry
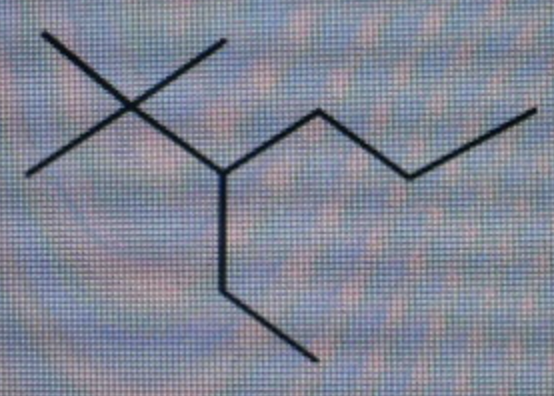
What is the IUPAC name for the compound shown below?
What is the IUPAC name for the compound shown below? Spelling and punctuation count!
Solutions
Expert Solution
Concepts and reason
IUPAC stands for the international union of pure and applied chemistry. IUPAC has given a nomenclature to name the organic compounds. The IUPAC name consists of three parts: root name, prefix, and suffix.
Fundamentals
The three parts of an IUPAC name are the root name, prefix, and suffix. The longest chain gives the root name. Prefix gives the position and name of the substitutions present on the longest chain. Suffix gives the functional group present in the structure.
The given structure of the compound is shown below.
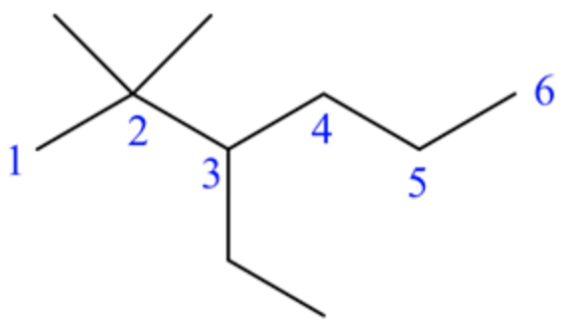
The longest chain is indicated by the numbers. The root name is hex.
The longest chain has six carbon atoms. So, the root name for the given structure is hex. Select the longest chain such that, the substituents have the lowest numbers.
Three substituents are present on the longest chain. They are two methyl groups and one ethyl group.
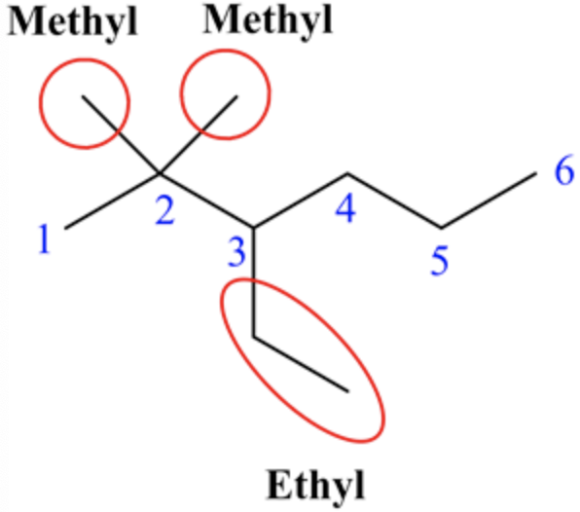
The prefix is 3-ethyl-2,2-dimethyl.
Two methyl groups are substituted at C-2 carbon and one ethyl group is substituted at C-3 carbon. So, the prefix is 3-ethyl-2,2-dimethyl. [Common mistake] Make sure that the substituents are named in alphabetical order.
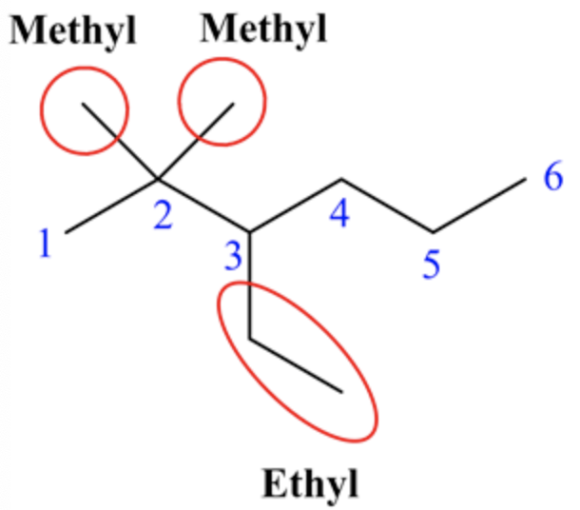
The suffix is ane.
Thus, the IUPAC name of the given structure is 3-ethyl- 2,2 -dimethylhexane.
Related Solutions
What is the IUPAC name for the compound shown below? Spelling and punctuation count!
What is the IUPAC name for the compound shown below? H3C-CH2-CH2-CH2-CH(-CH2-CH2-CH3)-CH2-CH2-CH3 (The parenthesis mean it is...
Give the IUPAC name for the following compound: Interactive MarvinView
The IUPAC-style name given below is not a correct name for a molecule. Draw the structure...
Including the cis or trans designation, what is the IUPAC name of the following substance?
What is the IUPAC name of the product formed by the following reactions: 1) 1-nitropropane is...
Give the IUPAC name for each of the following carboxylic acids.
1. Write the chemical structures of monomer, repeating unit and polymer ? IUPAC name, common name,...
The proton NMR spectrum for a compound with the formula C7H14O is shown below along with...
The following IUPAC name is incorrect. Correct it. 1,1-dimethyl-2-cyclohexene
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago