Question
In: Chemistry
How many lone pairs are on the central atom in BCl3?
A) How many lone pairs are on the central atom in BCl3?
Express your answer numerically as an integer.
B)How many lone pairs are on the central atom of BrF3?
Express your answer numerically as an integer.
Solutions
Expert Solution
Concepts and reason
Lone pair of electrons for an atom is defined as the non-bonding or unshared electrons in the atom's outermost orbit.
Generally, these lone pair of electrons is shown in the Lewis structure model of molecules. In Lewis structures, the non-bonding pair is located the central atom.
Fundamentals
Lewis structure: Lewis structure is a structure that shows all the bonding as well as non-bonding electrons present in it. Example:
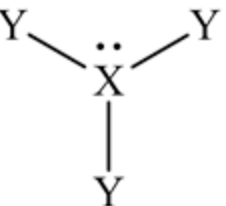
In this Lewis structure, the central atom is \(\mathrm{X}\), which has one lone pair in it and three bond pairs with 3 Y atoms.
$$ \begin{array}{|l|l|l|} \hline \begin{array}{l} \text { Valence electrons } \\ \text { (central atom) } \end{array} & \begin{array}{l} \text { Number of bonds } \\ \text { (sigma bonds) } \end{array} & \text { Number of lone pairs } \\ \hline \mathrm{P} & \mathrm{Q} & \frac{\mathrm{P}-\mathrm{Q}}{2} \\ \hline \end{array} $$
(A) The given molecule is \(\mathrm{BCl}_{3}\). In \(\mathrm{BCl}_{3}\) molecule, the central atom is boron (B). The atomic number for the boron atom is 5, and the valence electron for the boron atom is 3. The remaining chlorine atoms form sigma bonds with the boron atom, and therefore, the total number of sigma bonds present on the central atom is 3. The number of lone pairs present on the central metal atom is calculated below.
Number of lone pairs \(=\frac{(\text { valence electrons })-(\text { number of sigma bonds })}{2}\)
$$ \begin{array}{l} =\frac{(3)-(3)}{2} \\ =0 \end{array} $$
Lewis structure of the given molecule \(\mathrm{BCl}_{3}\) is shown below.
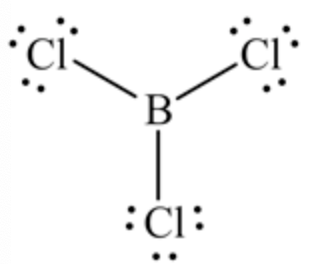
Therefore, the central boron atom has zero lone pair of electrons.
Part A The number of lone pair electron present on the central atom in \(\mathrm{BCl}_{3}\) is 0
Explanation | Common mistakes | Hint for next step
The central atom for the given molecule \(\mathrm{BCl}_{3}\) is boron, the atomic number of boron is 5, and the valence electrons in bromine atom are 3.
$$ \begin{array}{|l|l|l|} \hline \begin{array}{l} \text { Valence electrons } \\ \text { (central atom) } \end{array} & \begin{array}{l} \text { Number of bonds } \\ \text { (sigma bonds) } \end{array} & \text { Number of lone pairs } \\ \hline \mathrm{P} & \mathrm{Q} & \frac{\mathrm{P}-\mathrm{Q}}{2} \\ \hline \end{array} $$
Therefore, the central boron atom has zero lone pair of electrons.
(B) The given molecule is \(\mathrm{BrF}_{3}\). In \(\mathrm{Br} \mathrm{F}_{3}\) molecule, the central atom is bromine (Br). The atomic number for the bromine atom is \(35,\) and the valence electron for the bromine atom is 7. The remaining fluorine atoms form sigma bonds with the bromine atom, and therefore, the total number of sigma bonds present on the central atom is 3. The number of lone pairs present on the central atom is calculated below.
$$ \begin{array}{l} \text { Number of lone pairs }=\frac{(\text { valence electrons })-(\text { number of sigma bnds })}{2} \\ \qquad \begin{array}{l} =\frac{(7)-(3)}{2} \\ =\frac{4}{2} \\ =2 \end{array} \end{array} $$
Lewis structure of the given molecule \(\mathrm{BrF}_{3}\) is shown below.
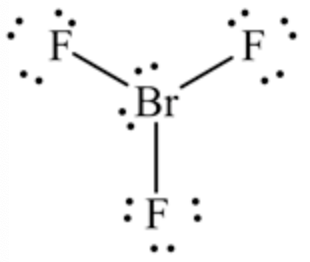
Therefore, the central bromine atom has two lone pairs of electrons.
Part B
The number of lone pair electron present on the central atom in \(\mathrm{Br} \mathrm{F}_{3}\) is 2.
The central atom for the given molecule \(\mathrm{BrF}_{3}\) is bromine, the atomic number of bromine is 35, and the valence electrons in the bromine atom are 7.
$$ \begin{array}{|l|l|l|} \hline \begin{array}{l} \text { Valence electrons } \\ \text { (central atom) } \end{array} & \begin{array}{l} \text { Number of bonds } \\ \text { (sigma bonds) } \end{array} & \text { Number of lone pairs } \\ \hline \mathrm{P} & \mathrm{Q} & \frac{\mathrm{P}-\mathrm{Q}}{2} \\ \hline \end{array} $$
Therefore, the central bromine atom has 2 lone pairs of electrons.
Part A
The number of lone pair electron present on the central atom in \(\mathrm{BCl}_{3}\) is 0.
Part B
The number of lone pair electron present on the central atom in \(\mathrm{BrF}_{3}\) is 2.
Related Solutions
How many lone pairs of electrons will be on the central atom in the correct Lewis structure for PBr3?
How many of the following have lone pairs on the central atom? NCl 3 SO 2...
1. Give the number of lone pairs around the central atom and the molecular geometry of...
Find point number of electron groups around central atom? Polarity? Number of Lone pairs for central...
Explain why only the lone pairs on the central atom are taken into consideration when predicting...
what is the 3D structure of XeF2 BCL3 and COCL2 with elements and lone pairs
In AX3, the central atom A forms three single covalent bonds and has two lone pairs....
Determine the following: Bonding pairs: lone pairs: Electron arrangement: BeF2 BCl3 SI6 CS2 CH2O
How many lone pairs are in the best Lewis structure of SeCN-?
Are the number of lone pairs of electrons on the central atoms the main factor that's...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago