Question
In: Chemistry
A.) Ammonium bisulfide, NH4HS, forms ammonia, NH3, and hydrogen sulfide, H2S, through the reaction NH4HS(s)⇌NH3(g)+H2S(g) 1.)This...
A.) Ammonium bisulfide, NH4HS, forms ammonia, NH3, and hydrogen sulfide, H2S, through the reaction
NH4HS(s)⇌NH3(g)+H2S(g)
1.)This reaction has a Kp value of 0.120 at 25 ∘C.
What is the initial pressure of H2S(g) in the flask?
Express your answer numerically in atmospheres.
B.) For the reaction
aA+bB⇌cC+dD
the reaction quotient, Q, is given by the expression
Q=[C]c[D]d[A]a[B]b
under any conditions. The value of Q is equal to Konly at equilibrium.
1.) The following reaction was carried out in a 2.75 L reaction vessel at 1100 K:
C(s)+H2O(g)⇌CO(g)+H2(g)
If during the course of the reaction, the vessel is found to contain 5.50 mol of C, 15.8 mol of H2O, 3.10 mol of CO, and 7.10 mol of H2, what is the reaction quotient Q?
Enter the reaction quotient numerically.
2.)The reaction
2CH4(g)⇌C2H2(g)+3H2(g)
has an equilibrium constant of K = 0.154.
If 7.00 mol of CH4, 4.00 mol of C2H2, and 10.15 mol of H2 are added to a reaction vessel with a volume of 5.40 L , what net reaction will occur?The reaction
has an equilibrium constant of = 0.154.
If 7.00 of , 4.00 of , and 10.15 of are added to a reaction vessel with a volume of 5.40 , what net reaction will occur?
| The reaction will proceed to the left to establish equilibrium. | |
| The reaction will proceed to the right to establish equilibrium. | |
| No further reaction will occur because the reaction is at equilibrium. |
C.)The following reaction was performed in a sealed vessel at 748 ∘C :
H2(g)+I2(g)⇌2HI(g)
Initially, only H2 and I2 were present at concentrations of [H2]=3.45M and [I2]=2.40M. The equilibrium concentration of I2 is 0.0400 M . What is the equilibrium constant, Kc, for the reaction at this temperature?
Solutions
Expert Solution
The reaction quotient determines the direction of the reaction. If the quotient reaction is less than the equilibrium constant, then the reaction will proceed to the right, if it is more than the equilibrium constant, the reaction will proceed to the left. If it is equal to the constant reaction, the system is in equilibrium.
So:
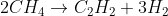
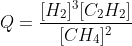
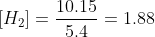
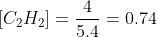
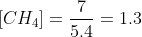
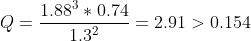
The reaction will proceed to the left to establish equilibrium.
Related Solutions
Ammonium bisulfide, NH4HS, forms ammonia, NH3, and hydrogen sulfide, H2S, through the reaction NH4HS(s)⇌NH3(g)+H2S(g) This reaction...
Ammonium bisulfide, NH4HS, forms ammonia, NH3, and hydrogen sulfide, H2S, through the reaction NH4HS(s)?NH3(g)+H2S(g) This reaction...
Ammonium bisulfide, NH4HS, forms ammonia, NH3, and hydrogen sulfide, H2S, through the reaction NH4HS(s)?NH3(g)+H2S(g) This reaction...
1) Ammonium bisulfide, NH4HS, forms ammonia, NH3, and hydrogen sulfide, H2S, through the reaction NH4HS(s)⇌NH3(g)+H2S(g) This...
± Heterogeneous Equilibrium of Ammonium Bisulfide - Copy Ammonium bisulfide, NH4HS, forms ammonia, NH3, and hydrogen...
At 22°C, Kp = 0.070 for the equilibrium: NH4HS (s) NH3 (g) + H2S (g) a)...
ITEM 17 The decomposition of NH4HS is endothermic: NH4HS(s)?NH3(g)+H2S(g) Which change to an equilibrium mixture of...
Consider the following reaction where Kc = 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2S(g) A...
Consider the following reaction where Kc = 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2S(g) A...
The decomposition of solid ammonium hydrogen sulfide to form ammonia gas and hydrogen sulfide gas is...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago