Question
In: Chemistry
You need to design a relief valve for a new pressurized propane storage tank. As part...
You need to design a relief valve for a new pressurized propane storage tank. As part of the design process, you need to calculate the maximum pressure inside the tank for any feasible “worst case” scenario. One of your design cases is that a fire breaks out beneath the tank, causing the liquid propane inside to rapidly heat up to 250°F. The tank is a rigid 10 ft3 vessel that holds 150 lbm of propane. What is the pressure inside the tank (in atm) for your fire case?
a. Using the ideal gas law
b. The van der Waals equation of state
c. The Peng-Robinson equation of state
Solutions
Expert Solution
a) PV = nRT
so P = nRT / V
and n = m/MM
first, we convert the lbm to grams:
1 lbm -------> 454 g: 150 * 454 = 68100 g
MM of propane (C3H8) = 12*3 + 8*1 = 44 g/mol
n = 68100 / 44 = 1547.73 moles
Now let's convert the temperature to K:
°C = 250 - 32 / 1.8 = 121.11 °C
K = 121.11 + 273 = 394.11 K
And the volume of ft3 to cm3 and then to mL and L:
10 ft3 * (30.48 cm/ft)3 * 1 mL/cm3 * 1 L/1000 mL = 283.17 L
now, using R = 0.0821 L atm / K mol we can use the ideal gas equation:
P = 1547.73 * 0.0821 * 394.11 / 283.17 = 176.85 atm
b) the Van der Waals equation is:
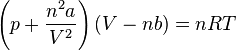
the values of a and b for propane are: 9.267 atm L2/mol and 0.089 L/mol so:
[P + 1547.732*9.267/283.172] (283.17 - 1547.73*0.089) = 1547.73*0.082*394.11
(P + 276.84)(145.42) = 50079.019
145.42P + 40258.072 = 50079.019
P = 50079.019 - 40258.072 / 145.42
P = 67.54 atm
I don't remember the Peng-Robinson equation, so I hope this helps.
Related Solutions
You are assigned the design of a cylindrical, pressurized water tank for a future colony on...
A cylindrical storage tank contains liquefied propane under a pressure of 1.5 MPa (gage pressure P=1.5...
A cylindrical storage tank contains liquefied propane under a pressure of 1.5 MPa (gage pressure P=1.5...
You work for a company that produces gas grills. A new design for a propane grill...
A storage tank contains ethanol. As liquid ethanol is taken from the lower part of the...
A spherical storage tank contains oil. The tank has a diameter of 6 feet. You are...
An oil company installs a new pipeline to connect a storage tank to an existing pump...
In case of big data there is a need of new data architecture, new storage approaches,...
You are assigned the responsibility to prepare a promotional campaign for a new allergy relief drug...
A.) A group of engineers developed a new design for a steel cable. They need to...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago