Question
In: Chemistry
1. Would a 50:50 solution of R and S enantiomers of the same compound have any...
1. Would a 50:50 solution of R and S enantiomers of the same compound have any effect on plane polarized light shining through it? Why or why not?
2. Based on your answer to the last question, how could you determine the enantiopurity of a given concentration of a sample if you know its specific rotation [α] using a polarimeter?
3. Give an example of a drug which can be sold as a racemic (R and S) mixture without any harmful effects.
Solutions
Expert Solution
1. There would be no net effect on plane-polarized light because of a 50:50 solution of R and S enantiomers of the same compound because racemic mixtures are optically inactive. This is because both R and S enantiomers would cancel each other's effects on the plane-polarized light. Let's assume, R enantiomer rotates the plane-polarized light in an anticlockwise direction (hypothetically) by an angle of 180o. Now, the other half of the mixture, S enantiomer would rotate the same plane-polarized light in the clockwise direction by an angle of 180o. So, after passing through the solution, there would be no net change in the light. Hence, the mixture would seem optically inactive.
2. The enantiopurity of a sample can be determined by calculating the enantiomeric excess of the sample by using the following formula:
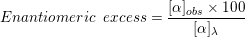
where,
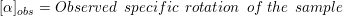
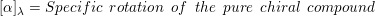
3. Examples of drugs which can be sold as a racemic (R and S) mixture are Ketamine, Ibuprofen, etc.
Related Solutions
Why is it not possible to assign R or S to a compound if it is...
(Compound value solving for r) At what annual rate would the following have to be invested?...
For any r, s ∈ N, show how to order the numbers 1, 2, . ....
1.Why would it be important to place the same amount of a solution in the polarimeter...
A. Prove that R and the real interval (0, 1) have the same cardinality.
a. In general is there any difference between R and S, and + and -? between...
a. In general is there any difference between R and S, and + and -? between...
True or false? 1) A 50% solution would contain 5000 mg/mL. 2) A solution with 500...
we have defined open sets in R: for any a ∈ R, there is sigma >...
Would the Rf of a given compound increase, decrease, or stay the same if you doubled...
- MINIMUM MAIN.CPP CODE /******************************** * Week 4 lesson: * * finding the smallest number * *********************************/...
- Do you think President Eisenhower had a successful presidency?
- Barbour Corporation, located in Buffalo, New York, is a retailer of high-tech products and is known...
- C PROGRAMMIMG I want to check if my 2 input is a number or not all...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
 queen_honey_blossom answered 2 months ago
queen_honey_blossom answered 2 months ago