Question
In: Chemistry
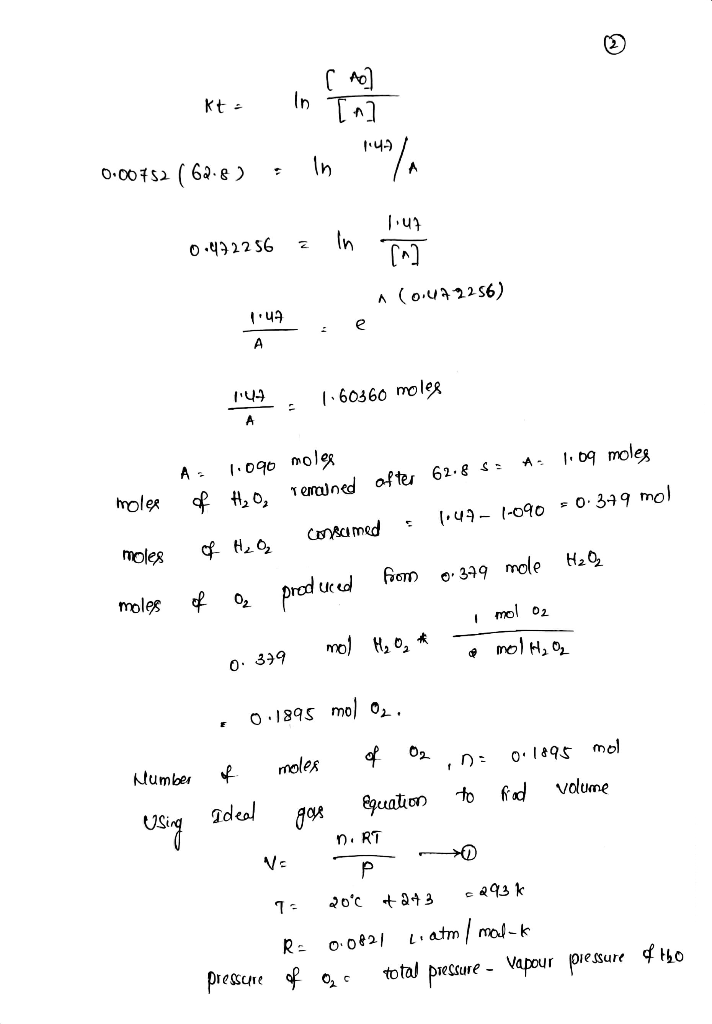
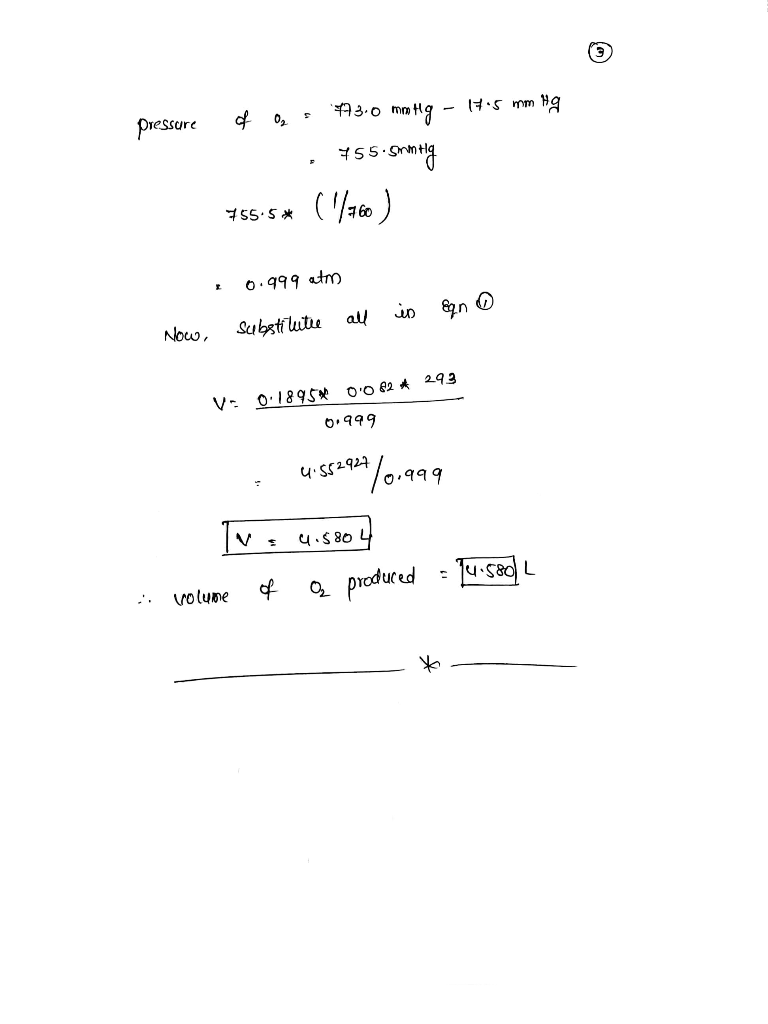
The reaction 2H2O2(aq)→2H2O(l)+O2(g)2H2O2(aq)→2H2O(l)+O2(g) is first order in H2O2H2O2 and under certain conditions has a rate constant...
The reaction 2H2O2(aq)→2H2O(l)+O2(g)2H2O2(aq)→2H2O(l)+O2(g) is first order in H2O2H2O2 and under certain conditions has a rate constant of 0.00752 s−1s−1 at 20.0 ∘C∘C. A reaction vessel initially contains 150.0 mLmL of 30.0% H2O2H2O2 by mass solution (the density of the solution is 1.11 g/mLg/mL). The gaseous oxygen is collected over water at 20.0 ∘C∘C as it forms.
What volume of O2O2 will form in 62.8 seconds at a barometric pressure of 773.0 mmHg . (The vapor pressure of water at this temperature is 17.5 mmHg)
Solutions
Related Solutions
The reaction 2H2O2(aq)→2H2O(l)+O2(g)is first order in H2O2 and under certain conditions has a rate constant of...
The reaction
2H2O2(aq)→2H2O(l)+O2(g)is first
order in H2O2 and under certain conditions has a rate constant of
0.00752 s−1 at 20.0 ∘C. A reaction vessel initially contains 150.0
mL of 30.0% H2O2 by mass solution (the density of the solution is
1.11 g/mL). The gaseous oxygen is collected over water at 20.0 ∘C
as it forms.
What volume of O2 will form in 80.9 seconds at a barometric
pressure of 771.4 mmHg ? (The vapor pressure of water at this
temperature...
CH 14 The reaction 2H2O2(aq)→2H2O(l)+O2(g) is first order in H2O2 and under certain conditions has a...
CH 14
The reaction
2H2O2(aq)→2H2O(l)+O2(g) is
first order in H2O2 and under certain conditions has a rate
constant of 0.00752 s−1 at 20.0 ∘C. A reaction vessel initially
contains 150.0 mL of 30.0% H2O2 by mass solution (the density of
the solution is 1.11 g/mL). The gaseous oxygen is collected over
water at 20.0 ∘C as it forms.
What volume of O2 will form in 83.2 seconds at a
barometric pressure of 725.2 mmHg . (The vapor pressure of water...
Hydrogen peroxide, H2O2H2O2, is used to disinfect contact lenses. 2H2O2(aq)→2H2O(l)+O2(g)2H2O2(aq)→2H2O(l)+O2(g) You may want to reference (Page)...
Hydrogen peroxide, H2O2H2O2, is used to disinfect contact
lenses.
2H2O2(aq)→2H2O(l)+O2(g)2H2O2(aq)→2H2O(l)+O2(g)
You may want to reference (Page) Section 6.1 while completing
this problem.
Part A
How many milliliters of O2(g)O2(g) at 27 ∘C∘C and 5.00 barrbarr
can be liberated from 18.95 mLmL of an aqueous solution containing
3.00%% H2O2H2O2 by mass? The density of the aqueous solution of
H2O2H2O2 is 1.01g/mLg/mL.
The reaction HCN (aq) + 2H2O (l) > NH4HCO2 (aq) is first order, and it's rate...
The reaction HCN (aq) + 2H2O (l) > NH4HCO2 (aq) is
first order, and it's rate =k [HCN]. The rate constant k at 65°C is
8.06 ×10 -8 s-1. How long will it take for thr concentration of the
HCN solution to drop from an initial 0.0800M to 0.0600M at this
temperature? What is the half life of thr reaction
the reaction 2 h2o2(aq) -> 2 h2o(l) + o2(g) is first order in h2o2 and has...
the reaction 2 h2o2(aq) -> 2 h2o(l) + o2(g) is first order in
h2o2 and has a rate constant of 0.00785 s- at 24C. a reation vessel
contains 190ml of 25% h2o2 by mass solution (density of the
solution is 1.09 g/ml). the gaseous oxygen is collected over water
at 24Cas it forms. What volume of o2 forms in 146 seconds at a
barometric pressure of 747.9 mmHg?
The reaction AB(aq)→A(g)+B(g) is second order in AB and has a rate constant of 0.0249 L⋅mol−1⋅s−1...
The reaction AB(aq)→A(g)+B(g) is second order in AB and has a
rate constant of 0.0249 L⋅mol−1⋅s−1 at 25.0 ∘C. A reaction vessel
initially contains 250.0 mL of 0.180 mol⋅L−1 AB which is allowed to
react to form the gaseous product. The product is collected over
water at 25.0 ∘C. Part A How much time is required to produce 270.0
mL of the products at a barometric pressure of 736.7 mmHg . (The
vapor pressure of water at this temperature is...
answer both pls 1. Consider the following half-reaction. 4H+(aq) + O2(g) + 4e- → 2H2O(l) Eo...
answer both pls
1. Consider the following half-reaction.
4H+(aq) + O2(g) + 4e- →
2H2O(l)
Eo = 1.23 V
What is E, if the pressure of oxygen gas is 1.0 atmosphere
and the pH is 2.92?
USE 2 DECIMAL PLACES. (Hint, are solids and pure liquids included
in Q? Using LeChatelier's principle is E going to be
larger or smaller?)
2. Mg2C2O4(s) →
2Mg2+(aq) +C2O42-(aq)
Ksp = 8.6 x 10-5
What is Qsp if 100 mL of 0.080 M
Mg(N03)2...
1. One of the half-reactions for the electrolysis of water is 2H2O(l) →O2(g) + 4H+(aq) +...
1. One of the half-reactions for the electrolysis of
water is
2H2O(l)
→O2(g)
+ 4H+(aq) +
4e−
If 0.992 L of O2 is collected
at 25°C and 755 mmHg, how many faradays of
electricity had to pass through the solution?
2.
What is
E°cell for the following reaction?
2 Ag(s) +
Sn2+(aq) → 2 Ag+(aq) +
Sn(s)
Ag+(aq) +
e– → Ag(s)
E° = 0.80 V
Sn4+(aq) +
2e– → Sn2+(aq)
E° = 0.13 V
Sn2+(aq) +
2e– → Sn(s)...
For the reaction NO + O3 → NO2 + O2 the second order rate constant has...
For the reaction NO + O3 → NO2 + O2 the second order rate
constant has a value of 1.8x10^-14 molecule-1 cm3 s-1 at 25°C. The
concentration of NO in a relatively clean atmosphere is 0.10 ppbv
(parts per billion by volume) and that of O3 is 15 ppbv. Calculate
these two concentrations in units of molecule cm-3. Calculate the
rate of the NO oxidation using concentration units of molecule
cm-3. Show how the rate law may be expressed in...
Consider the combustion of hydrochloric acid. 4 HCl (aq) + O2(g) 2H2O(l) + 2 Cl2(g)...
Consider the combustion of hydrochloric acid. 4 HCl (aq) + O2(g)
2H2O(l) + 2 Cl2(g) ΔHrxn = + 96.976 kJ/mol
B.If you place 750 mL of 0.500 M solution of HCl with 17.2 g of
O2, will any oxygen remain? (Hint: limiting reagent problem)
C.What mass of chlorine gas (in grams) is produced?
D) How many molecules of Cl2 are produced based on part (B)
E) What mass of excess reagent remains after the limiting
reagent is used up?...
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
ADVERTISEMENT

 queen_honey_blossom answered 3 months ago
queen_honey_blossom answered 3 months ago