Question
In: Chemistry
A. An unknown radioactive substance has a half-life of 3.20hours . If 10.6g of the substance...
A. An unknown radioactive substance has a half-life of 3.20hours . If 10.6g of the substance is currently present, what mass A0 was present 8.00 hours ago?
B. Americium-241 is used in some smoke detectors. It is an alpha emitter with a half-life of 432 years. How long will it take in years for 45.0{\rm \\%} of an Am-241 sample to decay?
C. A fossil was analyzed and determined to have a carbon-14 level that is 20{\rm \\%} that of living organisms. The half-life of C-14 is 5730 years. How old is the fossil?
Solutions
Expert Solution
A) t1/2 = 3.20hours
A= 10.6g
A0 = ?
t= 8 hours
t= n t1/2
8 = n x 3.2
n= 2.5
A= A0/2n
10.6 = A0/22.5
A0 = 59.96g (approximately 60g)
B) t1/2 = 432 years
for radio active decay
disntigration constant 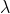 = 0.693/
t1/2
= 0.693/
t1/2
= 0.693/432
= 1.604 x 10^-3 year-1
now t= (2.303/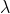 ) log (A0/A)
) log (A0/A)
= 2.303 x log (100/45) / 1.604 x 10^-3
= 497.9 year
C)
t1/2 = 5730 years
for radio active decay
disntigration constant 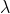 = 0.693/
t1/2
= 0.693/
t1/2
= 0.693/5730
= 1.2 x 10^-4 year-1
t= (2.303/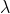 ) log
(100/20)
) log
(100/20)
= 2.303 x log (100/20) / 1.2 x 10^-4
= 13,414 years
fossil age =13,414 years
Related Solutions
An unknown radioactive substance has a half-life of 3.20 hours . If 16.0 g of the...
Cobalt- 60 is radioactive and has a half life of 5.26 years. Calculate the activity of...
The half-life of a radioactive isotope represents the average time it would take half of a...
Radioactive Decay Objective: to study the radioactive decay of materials. Understand the concept of half life and...
Suppose a radioactive nucleus has a half-life of 2 min. and suppose the counting rate at...
1.A radioactive sample has a half-life of 2.5 min. What fraction of the sample is left...
Carbon 14 (C-14), a radioactive isotope of carbon, has a half-life of 5730 ± 40 years....
1) The radioactive isotope 57Co has a half-life of 272 days. (a) Find its decay constant....
The half-life of a certain radioactive isotope is 3.24 years. If a sample consisting of 6.023x1023...
Example: The radioactive isotope decays by electron capture with a half –life of 272 days. •(a)...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 4 months ago
queen_honey_blossom answered 4 months ago