Question
In: Chemistry
compare 3 laws: van dear waals, redlich kwong equation, viral equation. which of the law do...
compare 3 laws: van dear waals, redlich kwong equation, viral
equation. which of the law do you think will model reality best for
N2 gas? And which of the law will best reality model for He
gas?
Solutions
Expert Solution
Vander Waals equation
The Van der Waals equation is :

 is molar volume, substance-specific
constants
is molar volume, substance-specific
constants 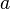 and
and 


Also written as


Redlich-Kwong equation



It is primarily due to its relatively simple form and While superior to the van der Waals equation of state
Virial equation of state[edit]
Main article: Virial expansion



virial equation is important due to the resion it can be derived directly from statistical mechanics

This expression is good for non-polar gases.
N2 is a nonpolar gas so virial equation model is most appropriae for N2 gas.
He is a noble gas which has vander wall bonding He, so the vander wall equation model is best suited for the He gas
Related Solutions
What is the role of the constants a and b in the van der waals equation...
What is the role of the constants a and b in the van der waals
equation in terms of the kinetic molecular theory?
Use the van der Waals equation and the ideal gas equation to calculate the volume of...
Use the van der Waals equation and the ideal gas equation to
calculate the volume of 1.000 mol of neon at a pressure of 500.0
bar and a temperature of 355.0 K. (Hint: One way to solve the van
der Waals equation for V is to use successive approximations. Use
the ideal gas law to get a preliminary estimate for V
V in ideal gas
V in van der waal gas
At high pressures, real gases do not behave ideally. (a) Use the van der Waals equation...
At high pressures, real gases do not behave ideally. (a) Use the
van der Waals equation and data in the text to calculate the
pressure exerted by 29.0 g H2 at 20 degree C in a 1.00 L container.
(b) Repeat the calculation assuming that the gas behaves like an
Ideal gas.
Derive constants for Van der Waals equation of state. Why is this equation used for? (Detailed...
Derive constants for Van der Waals equation of state. Why is
this equation used for? (Detailed explanation and derivation)
How can the Van Der Waals equation of state be used to determine whether a substance...
How can the Van Der Waals equation of state be used to determine
whether a substance will be a solid, liquid or gas at a certain
temperature and pressure?
1. Use the van der waals equation to calculate the pressure exerted by 1.470 mol of...
1.
Use the van der waals equation to calculate the pressure exerted by
1.470 mol of Cl2 in a volune of 5.285 L at a temperature of 280.0 K
.
2. Use the ideal gas equation to calculate the pressure
exerted by 1.470 mol of Cl2 in a volume of 5.285 L at a temperature
of 280.0 K .
H2O 5.537 5.465 0.0305 Use the van der Waals equation of state to calculate the pressure...
H2O
5.537
5.465
0.0305
Use the van der Waals equation of state to calculate the
pressure of 3.00 mol of H2O at 463 K in a 3.60 L vessel. Van der
Waals constants can be found here.Use the ideal gas equation to
calculate the pressure under the same conditions. Use Atm
The van der Waals equation for 1 mole of gas is given by (p +av-2)(v -...
The van der Waals equation for 1 mole of gas is given by (p
+av-2)(v - b) = RT. In general, curves of p versus v
forvarious values of T exhibit a maximum and a minimum at the
twopoints where (δp/δv)T = 0. The maximum and minumum
coalesceinto a single point on that curve
where(δ2p/δv2)T = 0 inaddition to
(δp/δv)T = 0. This point is calledthe "critical point"
of the substance and its temperature,pressure, and molar volume are
denoted by...
Describe how you would determine, using the van der Waals equation of state, the temperature of...
Describe how you would determine, using the van der Waals
equation of state, the temperature of a real gas mixture of 3
gasses (A,B and C) given the pressure P in the container, the mass
of each gas mA, mB, mC, and the
volume v of the container. You also have access to the critical
properties (TCA, TCB, TCC,
PCA, PCB, PCC) and molar masses
(MA, MB, MC,) of each gas. Use
these variables and list all equations and steps...
For oxygen gas, the van der Waals equation of state achieves its best fit for a=0.14...
For oxygen gas, the van der Waals equation of state achieves its
best fit for a=0.14 N⋅m^4/mol^2 and b=3.2×10^(−5) m^3/mol
1.Determine the pressure in 2.0 mol of the gas at 10 ∘C if its
volume is 0.24 L , calculated using the van der Waals equation.
2. Determine the pressure in 2.0 mol of the gas at 10 ∘C if its
volume is 0.24 L , calculated using the ideal gas law.
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
ADVERTISEMENT
 queen_honey_blossom answered 5 months ago
queen_honey_blossom answered 5 months ago