Question
In: Chemistry
1) How many photons are emitted during a transition from a higher level orbit to a...
1) How many photons are emitted during a transition from a higher level orbit to a lower level orbit? a)1 b)2 c)3 d)4
2) True/False? An electron can transition from any allowed orbit to any other allowed orbit.
3) What is the wavelength of light emitted when an electron drops from the 3 to the 2 shell in a hydrogen atom?
Solutions
Expert Solution
1.  E = E2-E1 =n
hV
E = E2-E1 =n
hV
 E = nhV n =1,2,3
....
E = nhV n =1,2,3
....
E2-E1 = hV
n=1
a.1 answer
2 . True
electron transition from higher energy level to lwer energy level and lower energy level to higher energy level.
3. 1/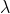 =
RH[1/n12 -1/n22]
=
RH[1/n12 -1/n22]
1/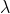 = 109677[1/22 --1/32]
= 109677[1/22 --1/32]
= 109677[0.25-0.11]
= 109677*0.14
= 15354.78 = 6.5*10-5 cm
Related Solutions
A) For hydrogen, find the frequency of light emitted in the transition from the 130th orbit...
A) For hydrogen, find the frequency of light emitted in
the transition from the 130th orbit to the 122th
orbit.
B) For hydrogen, find the frequency of light absorbed in
the transition from the 168th orbit to the 173th
orbit.
Please answer these three questions (1) In what ways are the photons emitted during stimulated emission...
Please answer these three questions
(1)
In what ways are the photons emitted during stimulated emission
related to the incident (stimulating) photons?
(2)
What conditions must a collection of atoms meet in order to amplify
an incident beam of light?
(3)
Very briefly describe how these requirements are met in a
helium
Although not visible through your spectroscope, photons of both higher and lower energy are being emitted...
Although not visible through your spectroscope, photons of both
higher and lower energy are being emitted from the hydrogen
discharge tube. Calculate one transition that would lead to the
emission of a UV (ultraviolet) photon. (UV light has a wavelengths
between approximately 4 and 400 nm). Of an IR (infared) photon? (IR
light has wavelengths between approximately 700 and 700,000
nm).
What is the frequency of the photons emitted by hydrogen atoms when they undergo transitions from...
What is the frequency of the photons emitted by hydrogen atoms
when they undergo transitions from n = 5 to n = 2? Calculate in s-1
In which region of the electromagnetic spectrum does this radiation
occur?
Topic: Spectroscopy (1 point) Calculate the photons emitted per second from an Alexa488 dye pumped at...
Topic: Spectroscopy
(1 point) Calculate the photons emitted per second from an
Alexa488 dye pumped at 100 W/cm 2 .
7. Suppose the number of photons emitted by an atom during a one-minute time window can...
7. Suppose the number of photons emitted by an atom during a
one-minute time window can be modeled as a Poisson random variable
with parameter ? = 2. Now, suppose you watch the atom for two
minutes, and the number of emitted photons in each minute is an
independent Poisson process. Calculate the probability that you see
exactly one photon during the two minutes. (Hint: this is the
probability that you see no photons in the first minute and one...
How many photons are contained in a burst of yellow light (589nm) from a sodium lamp...
How
many photons are contained in a burst of yellow light (589nm) from
a sodium lamp that contains 609 kJ of energy?
1. How many photons at 586 nm must be absorbed to melt 6.55 × 102 g...
1. How many photons at 586 nm must be absorbed to melt 6.55 ×
102 g of ice?
(It takes 334 J to melt 1 g of ice at 0° C. On average, how many
H2O molecules does one photon convert from ice to
water?
2. The highest speed limit in the United States
is 85.0 mph on an isolated stretch of rural interstate in Texas.
What is the speed limit in kilometers per hour? (1 mi = 1609 m)....
An electron in a hydrogen atom undergoes a transition from an orbit with quantum number ni to another with quantum number nf.
An electron in a hydrogen atom undergoes a transition from an orbit with quantum number ni to another with quantum number nf. Vi and Vf are respectively the initial and final potential energies of the electron. If Vi/ Vf = 6.25, then the smallest possible nf.
suppose that the microwave radiation has a wavelength of 11.6 cm . How many photons are...
suppose that the microwave radiation has a wavelength
of 11.6 cm . How many photons are required to heat 245 ml of coffee
from 25.0 °C to 62°C? Assume that the coffee has the same density
0.997 g/mL and specific heat capacity of 4.184 J/ (g•K), as water
over this temperature range.
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
ADVERTISEMENT
 queen_honey_blossom answered 1 year ago
queen_honey_blossom answered 1 year ago