Question
In: Chemistry
Under what circumstances can ?G be negative if ?G'
Under what circumstances can ?G be negative if ?G'
Solutions
Expert Solution
For a reaction at constant temperature and pressure, ?G in the Gibbs free energy is:
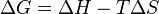
The sign of ?G depends on the signs of the changes in enthalpy (?H) and entropy (?S), as well as on the absolute temperature (T, in kelvin). ?G changes from positive to negative (or vice versa) where T = ?H/?S.
For heterogeneous systems where all of the species of the reaction are in different phases and can be mechanically separated, the following is true.
When ?G is negative, a process or chemical reaction proceeds spontaneously in the forward direction.
When ?G is positive, the process proceeds spontaneously in reverse.
When ?G is zero, the process is already in equilibrium, with no net change taking place over time.
We can further distinguish four cases within the above rule just by examining the signs of the two terms on the right side of the equation.
When ?S is positive and ?H is negative, a process is always spontaneous
When ?S is positive and ?H is positive, the relative magnitudes of ?S and ?H determine if the reaction is spontaneous. High temperatures make the reaction more favorable, because exothermicity plays a small role in the balance.
When ?S is negative and ?H is negative, the relative magnitudes of ?S and ?H determine if the reaction is spontaneous. Low temperatures make the reaction more favorable, because exothermicity is important.
When ?S is negative and ?H is positive, a process is not spontaneous at any temperature, but the reverse process is spontaneous.
Related Solutions
Under what circumstances can an employer act unilaterally?
Under certain circumstances, carbon dioxide, CO2(g), can be made to react with hydrogen gas, H2(g), to...
Under what circumstances Financial Liberalization can be beneficial for an economy?
under what circumstances can an illegal market be encouraged exist
Is sampling always necessary? Discuss under what circumstances it is necessary and under what circumstances it...
under what circumstances will the ramsey "optimal growth" discount rate be negative? Explain answer in terms...
under what circumstances can the supply curve shift up or down
(a) Under what circumstances can inventory be used as a hedge against inflation? (b) And what...
Under what circumstances can a man with type B blood and a woman with type a...
Under what circumstances can a college student qualify for the earned income credit?
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 queen_honey_blossom answered 1 year ago
queen_honey_blossom answered 1 year ago