Question
In: Physics
Ron Swanson is by his fireplace drinking his favorite Lagavulin scotch (the 16 year, of course)....
Ron Swanson is by his fireplace drinking his favorite Lagavulin scotch (the 16 year, of course). He pours 2 ounces (60 ml) of scotch taken from ambient temperature (20°C) and adds 2 cubes of ice, each weighing 1 g that are initially at 0°C. What is the final temperature of the scotch after the ice has melted and equilibrium has been reached? Include a heat diagram in your solution. Density of scotch = 950 kg/m3 Specific heat capacity: scotch 2000 J/(kg°C); water 4186 J/(kg°C) Latent heat of fusion for ice/water: 3.33 x 105 J/kg Important Note: you do NOT need to convert to K. If you use °C, the answer you will get will be in °C; if you convert to K, the answer you will get will be in K.
Solutions
Expert Solution
GIven,
Density of scotch, 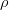 = 950 kg/m3 = [ 950*(103/106) ]
g/cm3
= 950 kg/m3 = [ 950*(103/106) ]
g/cm3
= 0.95 g/cm3
Volume of scotch, V = 60 ml
Since, 1 ml = 1 cm3
=> V = 60 cm3
Let mass of scotch be m
=> m = 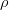 *V = 0.95 * 60 = 57 g
*V = 0.95 * 60 = 57 g
Now,
mass of ice, m' = 2 g
Now,
heat capacity of scotch, cs = 2000 J/kg C
= 2 J/g
C
= 2 J/g C
C
heat capacity of water, cw = 4186 J/kg C
= 4.186 J/g
C
= 4.186 J/g C
C
latent heat of fusion, L = 3.33 * 105
J/kg C
= 333 J/g
C
= 333 J/g C
C
Initial temperature, T = 20  C
C
Let the final temperature be T'
Now,
Heat lost by scotch in reaching the equilibrium temperature = latent heat needed to convert ice into water
+ heat needed to raise the temperature from 0 to T
=> m*cs*(20 - T) = m'*L + m'*cw*(T - 0)
=> 57*2*(20 - T) = 2*333 + 2*4.186*T
=> 2280 - 114*T = 666 + 8.372*T
=> 114*T + 8.372*T = 2280 - 666 = 1614
=> 122.372*T = 1614
=> T = 1614 / 122.372
= 13.2
 C
C
Thus, equlibrium temperature is 13.2  C
C
Related Solutions
ron who is 16 and studying fulltime whilst living with his parents 2017/18 tax year he...
1. Ron Remy enters into a three-year lease to rent his property. In the first year,...
Daniel is a 16 year old boy who is an identical twin. He and his brother...
Case Scenario Your favorite seventy-two-year-old Uncle Bob recently slipped on the ice and broke his left...
Uncle Fred recently died and left $340,000 to his 55-year-old favorite niece. She immediately spent $90,000...
Uncle Fred recently died and left $305,000 to his 50-year-old favorite niece. She immediately spent $90,000...
A 16-year-old male goes to his physician for an annual checkup. A routine urinalysis indicates the...
A 16-year-old Asian male presents at the doctor’s office complaining of “sores” on his arms and...
John is looking at several options to fund his son’s 4-year university degree. (16 marks) The...
4. John is a 16-year-old who loves to play soccer. His 18-year-old brother, Andre, passed out...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
 genius_generous answered 1 year ago
genius_generous answered 1 year ago