Question
In: Chemistry
10. What is the [CH3COOH ]/[CH3COO– ] ratio in an acetic acid/sodium acetate buffer at pH...
10. What is the [CH3COOH ]/[CH3COO– ] ratio in an acetic acid/sodium acetate buffer at pH = 4.90? (Ka = 1.8 x 10–5 )
a. 0.31
b. 0.70
c. 1.45
d. 2.41
e. 4.90
11. How many grams of NaCH3COO should be added to 250 mL of 0,100 M CH3COOH when the above solution is prepared?
a. 0.431 g
b. 2.95 g
c. 5.66 g
d. 1.43 g
e.11.9 g
Solutions
Expert Solution
10. Given
pH = 4.90 and Ka = 1.8 *10 -5
consider dissociation of acetic acid at equilibrium,
CH3COOH ⇌ CH3COO - + H+
now equilibrium constant Ka ={ [CH3COO– ] [ H+] } / [CH3COOH] ........equation 1
from pH we can calculate concentration of H+
pH = - log10 [ H+]
therfore [ H+] = antilog10 [ -pH ]
= antilog10 [ -4.90]
[ H+] = 1.25 * 10 -5
Now substituting value of Ka and H+ in eqution 1 we get
1.8 *10 -5 = [CH3COO– ] * 1.25 * 10 -5 / [CH3COOH]
but in problem we have been asked ratio of [CH3COOH] / [CH3COO– ] hence we rearrange above equation as follows:
[CH3COOH] / [CH3COO– ] = 1.25 * 10 -5 / 1.8 *10 -5
[CH3COOH] / [CH3COO– ] = 0.6944 which is nearly equal to 0.70 so option is b.
11.
Given
V = 250 ml = 0.250 l
C = 0.100 M
pH = 4.90
Ka = 1.8 *10-5
pKa of acetic acid = 4.75 from literature
Weight of CH3COONa ? in grams
We know Henderson–Hasselbalch equation as follows:
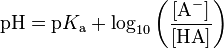
now substituting this values we get,
4.90 = 4.75 + log 10 ([salt] / [Acid] )
log 10 [salt] /[CH3COOH] = 4.90 - 4.75
[salt] /[CH3COOH] = antilog 10 (0.15)
[salt]/ [CH3COOH] = 1.4125
[salt] = 1.413 * 0.100
[salt] = 0.1413 M
we know concentration = moles / volume
moles = conc * volume = 0.1413 * 0.250 = 0.035325 moles
mass of salt = number of moles * molecular weight of CH3COONa
= 0.035325 * 82 = 2.8966 g nearly equal to 2.9 g so answer is b
Related Solutions
11- The pH of a sodium acetate-acetic acid buffer is 4.70. Calculate the ratio [CH3COO−] /...
Prepare 1 L of an acetic acid sodium acetate buffer with a pH of 5.00 and...
A buffer contains 0.150M acetic acid and 0.105M in sodium acetate. a) Calculate the pH of...
Preparation of the acetic acid-sodium acetate buffer: Calculate the theoretical pH of this buffer solution. 3.504...
1. A buffer solution consists of 0.25 M acetic acid (CH3COOH) and 0.10 M sodium acetate...
A 250.00 mL buffer solution is 0.250 M acetic acid (CH3COOH) and 0.100 M sodium acetate...
3. a) Calculate the pH of a sodium acetate-acetic acid buffer solution (Ka= 1.78 x 10-5)...
An acetic acid/ sodium acetate buffer solution is also 0.020 M AlCl3. At what minimum pH...
A 250mL buffer solution is 0.250M in acetic acid and 0.250M in sodium acetate. A) What...
A 280.0mL buffer solution is 0.220M in acetic acid and 0.220M in sodium acetate. A. What...
- C PROGRAMMIMG I want to check if my 2 input is a number or not all...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
 queen_honey_blossom answered 2 years ago
queen_honey_blossom answered 2 years ago