Question
In: Physics
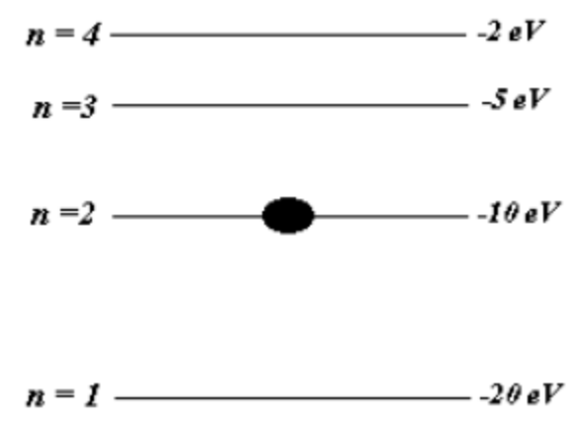
Below is a energy level scheme of a hypothetical one- electron element Mathematicum.
Below is a energy level scheme of a hypothetical one- electron element Mathematicum. The potential energy is taken to be zero for an electron at an infinite distance from the nucleus.
(a) (15) How much energy does it take to ionize an electron from the first excited level (n = 2) ?
(b) (4) An 8-eV photon is absorbed by a Mathematicum atom in its first excited level. As the atom returns to the first excited level, what possible energies can the emitted photons have? Assume there can be transitions between all pairs of levels, including sub-transitions back to first excited level.
(c) (15) What will happen to the atom if a photon of energy of5eV strikes a Mathematicum atom in its first excited state? Explain.
(d) (6) What will happen to the aton if a photon of energy of 7 eV strikes a Mathematicum atom in its first excited state? Explain.
(e) (15) EXTRA CREDIT: Photons emitted in the Mathemticum transitions n 3 to n = 2 and n 3 to n 1 will eject photoelectrons from a certain metal, but the photons emitted from the transitions n 4 to n = 3 will not, what are the limits (minimum and maximum values) of the work function of the metal?
Solutions
Expert Solution
a) energy of the first excited state = -10eV
so energy of 10eV is needed to ionize
b) the possible transitions are
n=4 to n=3 energy given out = 5 -2 = 3eV
n= 4 to n=2 energy given out = 10 -2 = 8eV
n= 3 t0 n=2 energy given out = 10 -5 = 5eV
hence photons with energies of 3eV, 5eV and 8 eV can be emitted
c) if a photon of 5eV is absorbed , the electron would jump to second excited state that is n=3
d) the photon would not be absorbed as a photon can not be partially absorbed
e) the minimum value of work function is 5 eV
and maximum value of work function is 15eV
Related Solutions
Energy diagram for an atom contains an excited electron at n = 4 level (see below...
Energy diagram for an atom contains an excited electron at n = 4
level (see below table). Calculate the longest wavelength of light
that is emitted.
Answer must be in meters!
Energy Level (n)
Energy
n=1
1.13 x 10-27 J
n=2
2.05 x 10-27 J
n=3
2.40 x 10-27 J
n=4
2.99 x 10-27 J
n=5
3.97 x 10-26 J
Please thoroughly explain every step and equation as I've been
attempting this question for hours and still do not understand.
In a hydrogen atom, how might an electron move from one energy level to another?
In a hydrogen atom, how might an electron move from one energy
level to another?
The energy required to remove an electron from a surface of a solid element is called...
The energy required to remove an electron from a surface of a
solid element is called its work function.
If a minimum of 226.7 kJ/mol is required to remove electrons from
Li atoms on a surface of a sample of lithium, what is the maximum
wavelength (λmax) of light that can remove an
electron from a Li atom on this surface?
5.277×102 nm
1pts
You are correct.
Your receipt no. is 155-2060
Previous Tries
If the same lithium surface is...
Calculate the energy change when an electron falls from the n =6 energy level to the...
Calculate the energy change when an electron falls from the n =6
energy level to the n = 1 energy level in a hydrogen atom.
ΔE=−2.178×10−18 J ( 1/n^2 final)- (1/n^2 initial)
How many orbitals are described by each of the below
combinations of quantum numbers?
n = 3, ℓ =2
n = 4, ℓ = 2, mℓ = 2
Choose one:
A. orbitals that randomly fill, but that are always
identical.
B. orbitals of the same shape.
C. orbitals...
For the element Na, what is the greatest energy level and how many electrons are in...
For the element Na, what is the
greatest energy level and how
many electrons are in this energy level?
Is it more beneficial to gain or lose electrons to
obtain noble gas configuration?
What would be the charge on the
ion?
Discuss the reduced zone-scheme for representing electron energy band structures and show how the periodic zone...
Discuss the reduced zone-scheme for representing electron energy
band structures and show how the periodic zone scheme and the
reduced zone-schemes are equivalent.
The ionization energies for a hypothetical element are listed below. Based on these ionization energies, identify...
The ionization
energies for a hypothetical element are listed below. Based on
these ionization energies, identify which group the element would
be from on the Periodic Table.
IE1
(kJ/mol)
IE2
(kJ/mol)
IE3
(kJ/mol)
IE4
(kJ/mol)
IE5
(kJ/mol)
IE6
(kJ/mol)
600
1200
7300
9580
12,600
14,640
In the energy pyramid below, calculate the amount of energy that is passed up from one trophic level to the next, assuming only 10% of the energy from the previous level is available for the next level.
In the energy pyramid below, calculate the amount of energy that is passed up from one trophic level to the next, assuming only 10% of the energy from the previous level is available for the next level. For each trophic level, circle all the words that apply to identify each organism as either a producer or consumer and as either an autotroph or a heterotroph. If the organism could be considered a predator and/or prey, circle those words also. Questions 1. Assume...
Calculate the effective nuclear charge for an electron in the fourth energy level of a neutral...
Calculate the effective nuclear charge for an electron in the
fourth energy level of a neutral atom of copper and bromine. How do
these values explain the difference in atomic radii for copper vs.
bromine? Which element has the larger atomic radius?
If a single electron in an excited hydrogen atom is occupying the 3rd energy level and...
If a single electron in an excited hydrogen atom is
occupying the 3rd energy level and then relaxes back to
the ground state, how much energy is released in the form of
electromagnetic radiation?
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
- A bicycle wheel has a diameter of 63.9 cm and a mass of 1.86 kg. Assume...
- Cane Company manufactures two products called Alpha and Beta that sell for $150 and $110, respectively....
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
ADVERTISEMENT
 genius_generous answered 3 years ago
genius_generous answered 3 years ago