Question
In: Chemistry
Calcium carbide crystallizes as a cube with 1 calcium ion on each corner and one calcium...
Calcium carbide crystallizes as a cube with 1 calcium ion on each corner and one calcium ion in the center of the cell. There are 8 carbon atoms located along edges of the cell and there are two carbons internal to the cell. What is the formula of calcium carbide?
Ca2C3
CaC4
CaC3
CaC2
CaC
Solutions
Expert Solution
- A cube has 8 corners and 12 edges. In a cubic unit cell, 8
atoms at the corners are shared by the 8 neighbouring cells and
atom at each edge is shared by 4 neighbouring atoms.
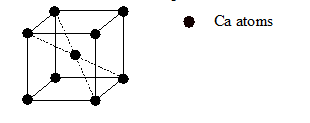
- Therefore, Calcium atoms at corners that belong to one unit cell =

- 1 Ca atom is at centre that completely belongs to that one unit cell.
- Total number of Ca atoms in a unit cell = 1+ 1 = 2
- Now there are 8 carbon atoms along edges of the cell. Thus, C atoms that belongs to one unit cell is

- Additionally, there are two carbon atoms present in the cell. Therefore, total number of C atoms in a unit cell = 4
- Hence, the formula for calcium carbide is Ca2C4 or CaC2.
- Therefore, the correct option is CaC2.
Related Solutions
a. What is the length of the line (labeled c) that runs from one corner of the cube diagonally through the center of the cube to the other corner in terms of r (the atomic radius)?
Consider the body-centered cubic (BCC) structure in Figure 3.
a. What is the length of the line (labeled c) that runs from one corner of the cube diagonally through the center of the cube to the other corner in terms of r (the atomic radius)?
b. Use the Pythagorean theorem to derive the expression for the length of the line (labeled b) that runs diagonally across one the faces of the cube in terms of the edge length (l).
c. Use the answer...
A cube has sides of length L = 1.00 mm. One corner is at the origin....
A cube has sides of length L = 1.00 mm. One corner is at the
origin. The nonuniform electric
field is given by E~ = (19.00N/C ·m) x ˆi−(1.34N/C ·m) z
ˆk. (a) find the electric flux through
each of the six cube faces S1, S2, S3, S4, S5, S6. (b) Find the
total electric charge inside the
cube.
What products result when calcium carbide is combined with water?
What products result when calcium carbide is combined with
water?
4. Calcium ion forms a weak 1:1 complex with nitrate ion with a formation constant of...
4. Calcium ion forms a weak 1:1 complex with nitrate ion with a
formation constant of 2.0. What would the equilibrim concentrations
of Ca2+ and Ca(NO3)+ in a solution
prepared by adding 10.0 mL each of 0.010 M M CaCl2 and
2.0 M NaNO3? (neglect diverse ion
effects).
Show all work please
A quantity of 2.00 g of calcium carbide is reacted at 500.0 K with excess of...
A quantity of 2.00 g of calcium carbide is reacted at 500.0 K
with excess of liquid water in a closed container fitted with a
piston. The only two products of this reaction are ethyne and
calcium hydroxide calculate the work done by the production
of C2H2 gas against an external pressure assume the
reaction proceeds until the pressure of the gas produced equals the
external pressure (atmospheric pressure). Assume that the reaction
proceeds until the pressure of the gas...
A mud logger places a sample of calcium carbide in the drillstring when a connection is...
A mud logger places a sample of calcium carbide in the
drillstring when a connection is made. The calcium carbide reacts
with the mud to form acetylene gas. The acetylene gas is detected
by a gas detector at the shale shaker after pumping 4, 500 strokes.
The drill string is composed of 9, 500 ft of 5-in, 19.5-lbm/ft
drill pipe and 500 ft of drill collars having an ID of 2.875 in.
The pump is a double acting duplex pump...
What is the functions of the following: bicarbonate ion calcium ion chloride ion magnesium ion phosphate...
What is the functions of the following:
bicarbonate ion
calcium ion
chloride ion
magnesium ion
phosphate ion
potassium ion
sodium ion
sulfate ion
Thank you
Arrange the following ions in order of increasing ionic radius: 1.) potassium ion, calcium ion, sulfide...
Arrange the following ions in order of increasing ionic
radius:
1.) potassium ion, calcium ion, sulfide ion, chloride ion
2.)barium ion, iodide ion,
telluride ion, cesium ion
Acetylene, C2H2 (boiling point = –84°C), is produced by the reaction of solid calcium carbide, CaC2,...
Acetylene, C2H2 (boiling point = –84°C),
is produced by the reaction of solid calcium carbide,
CaC2, and liquid water. Calcium hydroxide solution is
the other product of the reaction. 127 kJ of energy is transferred
to the atmosphere per mole of acetylene reacted. What mass of
calcium carbide in kilograms and volume of water in gallons are
needed to produce 125 cubic feet of acetylene at 17°C and 1.0 bar?
What quantity of energy in megajoules will be transferred in...
Explain the relation between muscles, ATP energy, and the calcium ion
Explain the relation between muscles, ATP energy, and the
calcium ion
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
ADVERTISEMENT

 queen_honey_blossom answered 3 years ago
queen_honey_blossom answered 3 years ago