Question
In: Biology
During acetyl CoA formation and the citric acid cycle, all of the carbon atoms that enter cellular respiration in the glucose molecule are released in the form of CO2.
Part A - Carbon atoms in acetyl CoA formation and the citric acid cycle
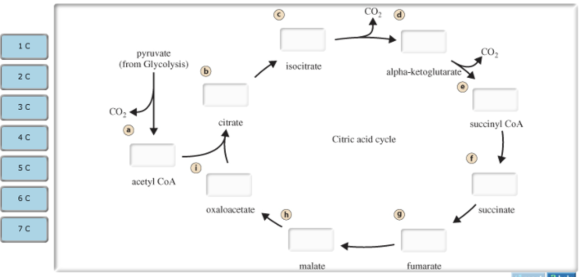
During acetyl CoA formation and the citric acid cycle, all of the carbon atoms that enter cellular respiration in the glucose molecule are released in the form of CO2. Use this diagram to track the carbon-containing compounds that play a role in these two stages.
Drag the labels from the left (which represent numbers of carbon atoms) onto the diagram to identify the number of carbon atoms in each intermediate in acetyl CoA formation and the citric acid cycle. Labels may be used more than once.
Part B - Net redox reaction in acetyl CoA formation and the citric acid cycle
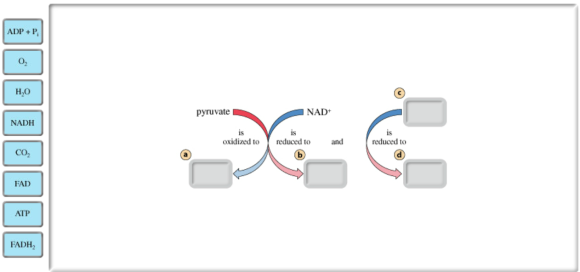
In the sequential reactions of acetyl CoA formation and the citric acid cycle, pyruvate (the output from glycolysis) is completely oxidized, and the electrons produced from this oxidation are passed on to two types of electron acceptors.
Drag the labels on the left to show the net redox reaction in acetyl CoA formation and the citric acid cycle. Note that two types of electron carriers are involved.
Part C - Why is the citric acid cycle a cyclic pathway rather than a linear pathway?
In the oxidation of pyruvate to acetyl CoA, one carbon atom is released as \(\mathrm{CO}_{2}\). However, the oxidation of the remaining two carbon atoms-in acetate- -to \(\mathrm{CO}_{2}\), requires a complex, eight-step pathway-the citric acid cycle. Consider four possible explanations for why the last two carbons in acetate are converted to \(\mathrm{CO}_{2}\) in a complex cyclic pathway rather than through a simple, linear reaction.
- Use your knowledge of the first three stages of cellular respiration to determine which explanation is correct.
- More ATP is produced per \(\mathrm{CO}_{2}\), released in cyclic processes than in linear processes.
- It is easier to remove electrons and produce \(\mathrm{CO}_{2}\) from compounds with three or more carbon atoms than from a two-carbon compound such as acetyl CoA.
- Redox reactions that simultaneously produce \(\mathrm{CO}_{2}\) and NADH occur only in cyclic processes.
- Cyclic processes, such as the citric acid cycle, require a different mechanism of ATP synthesis than linear processes, such as glycolysis.
Solutions
Expert Solution
Answer A:
a. Acetyl CoA -2C
b. Citrate -6C
c. Isocitrate -6C
d. alpha-ketoglutarate -5C
e. Succinyl CoA -4 C
f. Succinate -4C
g. Fumarate -4C
h. Malate -4C
i. Oxaloacetate -4C
Answer B:
a. \(\mathrm{CO}_{2}\)
b. NADH
C. FAD
d. FADH \(_{2}\)
Answer C:
Option \(\mathrm{b}\) - It is easier to remove electrons and produce \(\mathrm{CO}_{2}\) from compounds with three or more carbon atoms than from a two-carbon compound such as acetyl CoA.
Acetyl CoA can be also be directly oxidized to two molecules of \(\mathrm{CO}_{2}\), however, energetically, it is more economical for the cell to add acetyl CoA to citrate and then oxidize it.
Related Solutions
After all the CO2 is released during the Citric acid cycle, what is the point of...
Paragraph form Compare and contrast the reactions of the Citric Acid Cycle of Cellular Respiration with...
Assuming that all the 14C-labeled Acetyl-CoA formed enters the citric acid cycle at the same time,...
CELLULAR RESPIRATION 1- Steps of Cellular Respiration: Anaerobic vs. Aerobic a. Glycolysis b. Citric acid cycle...
What conditions are required to obtain energy from acetyl CoA in the citric acid cycle? Select...
CO2 is a byproduct of cellular respiration. When does it form? a. intermediate step b. citric...
Glucose Catabolism 1) Aerobic cellular respiration Glycolysis, Citric Acid cycle, Electron Transport Chain etc. 2) Anaerobic...
Which of the following stages of cellular respiration makes the most ATP? glycolysis citric acid cycle...
6.Select the process of cellular respiration in the correct chronological order glycolysis > citric acid cycle...
What are the main products/reactants/steps for each: Glycolysis is part of cellular respiration: Citric Acid cycle...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
- How many grams are in a 0.10 mol sample of ethyl alcohol?
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago