Question
In: Chemistry
Give the product expected when the following alcohol reacts with pyridinium chlorochromate (PCC).
Give the product expected when the following alcohol reacts with pyridinium chlorochromate (PCC).
(Assume that PCC is present in excess.)
Solutions
Expert Solution
Concept and reason
The concept used is to predict a carbonyl compound's formation by the reaction between alcohol and excess pyridinium chloride. Pyridinium chloride is an oxidizing agent that oxidizes the alcohol group into the 1carbonyl group.
Fundamentals
The \(-\mathrm{OH}\) group in the reactant will decide the carbonyl compound formed in the reaction. \(1^{\circ}-\mathrm{OH}\) are oxidized to aldehydes by PCC. \(2^{\circ}-\mathrm{OH}\) are oxidized to ketones by \(\mathrm{PCC}\) \(3^{\circ}-\mathrm{OH}\) produce no reaction by the oxidation of \(\mathrm{PCC} .\) Reaction of alcohols with PCC forms a carbonyl compound.
Consider the given reactant as follows:
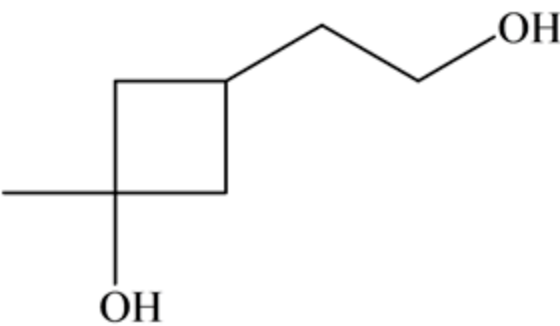
Here in this reactant, there are two \(-\mathrm{OH}\) groups. The reaction is as follows:
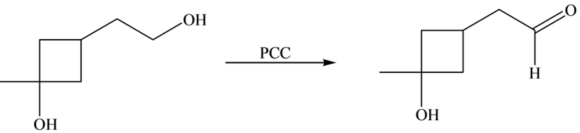
The \(1^{\circ}-\mathrm{OH}\) alcohol group that is present on the ethyl substituent is oxidized to the aldehyde group. [Hint for the next step] Identify the product of the reaction by the oxidation of \(3^{\circ}-\mathrm{OH}\) group.
The reaction is as follows:
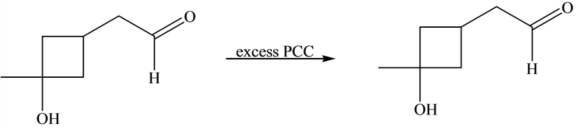
Part 1 Therefore, the product of the reaction is as follows:

The \(-\mathrm{OH}\) group that is directly attached to the cyclobutane ring is a \(3^{\circ}-\mathrm{OH}\) group. Although excess \(\mathrm{PCC}\) is present, there is no reaction on this \(3^{\circ}-\mathrm{OH}\) group.
Related Solutions
Give mechanism and product expected when 1-hexyne is treated with NaNH2 in liquid ammonia.
When a solution of barium hydroxide reacts with a solution of phosphorous acid, the product are...
When methyl alcohol is treated with NaH, the product is CH3O -Na+ (and H2) and not...
Draw the alcohol that is oxidized to give the product shown below. Show all hydrogen atoms.
Write the equation showing the formation of a monosubstituted product when hexane reacts with chlorine. Use...
What is the mass of each product when 150.0 g of pentene (C5H10) reacts with excess...
A mixture of AgNO3, NH4OH reacts with which of the following class of compounds to give...
When alkenes are hydrated, the final product is an alcohol, but alkyne hydration produces carbonyls. Write...
What is the percent yield of the solid product when 12.01 g of iron(III) nitrate reacts...
draw the reaction between isobutyl alcohol and propyl alcohol. give the common and IUPAC names of...
- What’s the cost of each component of capital and which need to be adjusted? What do...
- Answer the following questions 1) How does ASC 606 — Revenue From Contracts With Customers(new standard...
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago