Question
In: Chemistry
Classify each orbital diagram for ground-state electron configurations by the rule or principle it violates.
Part A
Classify each orbital diagram for ground-state electron configurations by the rule or principle it violates.
Drag the appropriate items to their respective bins.
Solutions
Expert Solution
Concepts and reason
This problem is based on three basic rules of filling of electrons in the atomic shells and subshells. Their rules are Aufbau rule, Hund's rule, and Pauli's exclusion principle.
Fundamentals
Aufbau rule: It states that the subshells are arranged in increasing order of their \(\mathrm{n}+\mid\) value where \(\mathrm{n}\) is the number of main shell and \(l\) is the number of subshell. Hund's rule: Pairing of electrons in subshells of same energy starts only after each subshell is occupied by at least one electron.
Pauli's exclusion principle: Any two electrons present in an atom cannot have the same set of all the four quantum numbers.
The following orbital diagrams violate Aufbau rule.
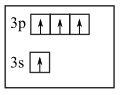
In first diagram, n + l value of 3p is 3 + 1 = 4
Whereas n + l value of 3s is 3 + 0 = 3
So, 3s should be filled first not 3p
In second diagram, n + l value of 3d is 3 + 2 = 5
n + l value of 4p is 4 + 2 = 6
n + l value of 4s is 4 + 0 = 4
so filling order should be 4s<3d<4p
The following orbital diagrams violate Hund’s rule.
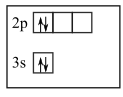
In the first diagram, there is the pairing of electrons while orbitals are still empty.
In the second diagram, singly present electrons are of opposite spins which is against Hund’s rule.
The following orbital diagrams violate Pauli’s rule.
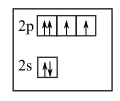
The first two electrons in \(2 p\) orbital have same set all four quantum numbers that is \(n=2,1=1, m_{l}=-1, s=\) \(m_{s}=+\frac{1}{2}\) Which is in violation to Pauli's exclusion principle.
Related Solutions
What is the ground state electron orbital diagram for Chromium?
Portions of orbital diagrams representing the ground-state electron configurations of certain elements are shown here: ?...
The following electron configurations represent excited states. Identify the element and enter its ground-state condensed electron...
Write the ground state electron configurations for the following. Use the noble gas shorthand notation. List...
Write the complete electron configurations Cobal(Co) atom for its: 1-its ground state 2- an...
Write the electron configurations and orbital spin diagrams for the neutral atom and the kon of...
The observed electron configurations for Cr and Cu both occur by moving an electron from the orbital of a p sublevel to an orbital of the d sublevel.
Use the aufbau principle to obtain the ground-state, noble gas core, electron configuration of selenium. Then,...
The electron configurations described in this chapter all refer to gaseous atoms in their ground states....
What is electron configuration and the orbital diagram for the element Mercury ?
- Project 7-6: Sales Tax Calculator Create a program that uses a separate module to calculate sales...
- On June 30, Sharper Corporation’s stockholders' equity section of its balance sheet appears as follows before...
- In this journal you are asked to take the role of a mayor or congressional representative...
- Answer correctly the below 25 multiple questions on Software Development Security. Please I will appreciate the...
- 1. The activation energy of a certain reaction is 41.5kJ/mol . At 20 ?C , the...
- Give TWO pieces of evidence that you've successfully made methyl salicylate. Remember when you cite TLC...
- Describe briefly the evolution of Craniata and Vertebrata.
 Dr. OWL answered 5 years ago
Dr. OWL answered 5 years ago