Question
In: Physics
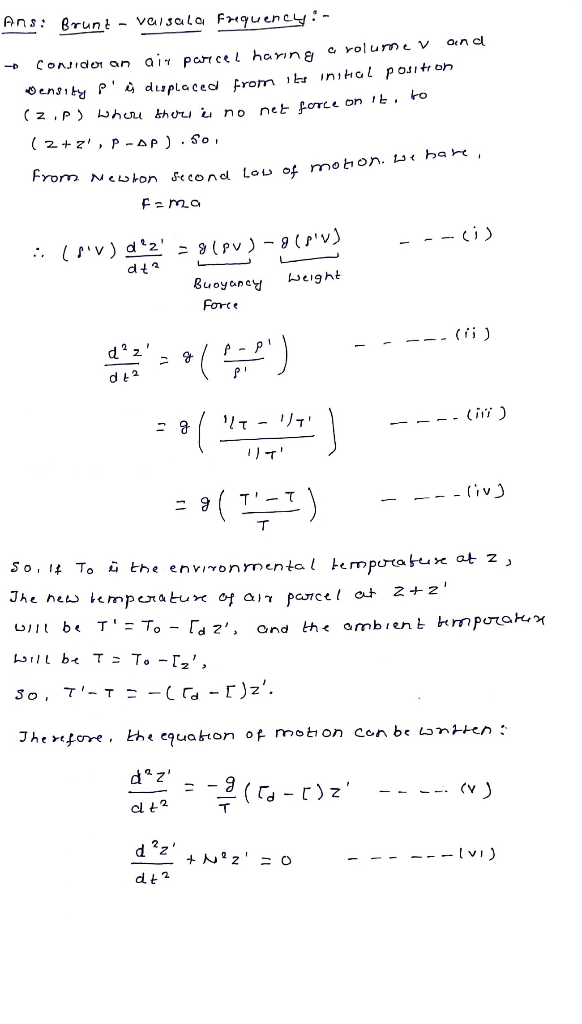
Calculate Brunt-Vaisala Frequency for a neutrally stable dry atmosphere
Calculate Brunt-Vaisala Frequency for a neutrally stable dry atmosphere
Solutions
Related Solutions
Without using Pivot Tables, calculate the Frequency, Relative Frequency, and Percent Frequency for each major. Major...
Without using Pivot Tables, calculate the Frequency, Relative
Frequency, and Percent Frequency for each major.
Major
ECON
BIOL
CHEM
CHEM
MKTG
CHEM
ACCT
CHEM
MGMT
BIOL
BIOL
IBUS
CHEM
ACCT
FINA
CHEM
MKTG
CHEM
PHYS
ECON
PHYS
ECON
MKTG
ACCT
MATH
IBUS
MATH
MGMT
ACCT
ECON
BIOL
CHEM
PHYS
MGMT
ACCT
ACCT
CHEM
PHYS
ECON
ACCT
MGMT
ECON
MGMT
MATH
ACCT
MATH
MGMT
MATH
FINA
ACCT
CHEM
MGMT
ECON
ECON
FINA
IBUS
MGMT
FINA
MGMT
CHEM
ACCT
ACCT
IBUS
MKTG...
The partial pressure of argon in the atmosphere is 7.10torr . Calculate the partial pressure in...
The partial pressure of argon in the atmosphere is 7.10torr .
Calculate the partial pressure in mmHg and atm . Round each of your
answers to 3 significant digits.
How to calculate frequency response of a pressure transducer?
How to calculate frequency response of a pressure
transducer?
The atmosphere is 0.9% Argon. Calculate the concentration of Argon in atmospheric air at a temperature...
The atmosphere is 0.9% Argon. Calculate the concentration of
Argon in atmospheric air at a temperature of 286 K and pressure of
998 mBar
Based on the Earth’s mean surface pressure, calculate the weight of the atmosphere in kg to...
Based on the Earth’s mean surface pressure, calculate the weight
of the atmosphere in kg to 1 significant figure. Show your
work.
(a) Calculate BE/A (in MeV) for 12C. Stable and relatively tightly bound, this nuclide is most...
(a)
Calculate BE/A (in MeV) for 12C. Stable and
relatively tightly bound, this nuclide is most of natural carbon.
(Assume 1 u = 931.5 MeV/c2. Give your answer to
at least 2 decimal places.)
answer in MeV
(b)
Calculate BE/A (in MeV) for 11C.
answer in MeV
Is the difference in BE/A between 12C and
11C significant? One is stable and common, and the other
is unstable and rare. (Assume the difference to be significant if
the percent difference between...
Following the soxhlet procedure, calculate the % fat content on dry and wet basis of a...
Following the soxhlet procedure, calculate the % fat content on
dry and wet basis
of a cheese sample considering the following:
Weight of dried sample = 2.0251 g
Weight of sample + thimble + glass wool = 5.4047 g
Weight of defatted sample + thimble + glass wool = 4.4107 g
% moisture = 35%
1 Larmor radius and gyration frequency (10 P) Calculate the Larmor radius and the gyration frequency...
1 Larmor radius and gyration frequency (10 P) Calculate the
Larmor radius and the gyration frequency (4 P) for:
1. An electron in the Earth’s ionosphere at 300 km altitude,
where the magnetic flux density is B ≈ 0.5 · 10−4 T, considering
that the electron moves at the thermal velocity (p kT /m) with T =
1000 K. (1 P)
2. An oxygen ion O+ in the Earth’s ionosphere for the same
conditions as above. Is there a difference...
Calculate the number of pounds of CO 2 released into the atmosphere when a 21.0 gallon...
Calculate the number of pounds of CO 2 released into the
atmosphere when a 21.0 gallon tank of gasoline is burned in an
automobile engine. Assume that gasoline is primarily octane, C 8 H
18 , and that the density of gasoline is 0.692 g ⋅ mL − 1 . This
assumption ignores additives. Also, assume complete combustion.
Useful conversion factors: 1 gallon = 3.785 L 1 kg = 2.204 lb
Calculate the number of pounds of CO2 released into the atmosphere when a 25.0-gallon tank of...
Calculate the number of pounds of CO2 released into the
atmosphere when a 25.0-gallon tank of gasoline is burned in an
automobile engine. Assume that gasoline is primarily octane, C8H18,
and that the density of gasoline is 0.692 g·mL–1 (this assumption
ignores additives). Also assume complete combustion.
ADVERTISEMENT
ADVERTISEMENT
Latest Questions
- C PROGRAMMIMG I want to check if my 2 input is a number or not all...
- In long paragraphs answer the questions below: Discuss the key components (where, when, what) and causes...
- Sinkal Co. was formed on January 1, 2018 as a wholly owned foreign subsidiary of a...
- Larry’s best friend, Garfield, owns a lasagna factory. Garfield’s financial skills are not very strong, so...
- Redox/Oxidation lab with Metals and Halogens So basically we were testing different reactions and observing changes....
- CORAL LANGUAGE ONLY Write a function DrivingCost with parameters drivenMiles, milesPerGallon, and dollarsPerGallon, that returns the...
- do you believe, as bonilla-silva does, that convert forms of racism are widespread? why or why...
ADVERTISEMENT

 genius_generous answered 1 month ago
genius_generous answered 1 month ago